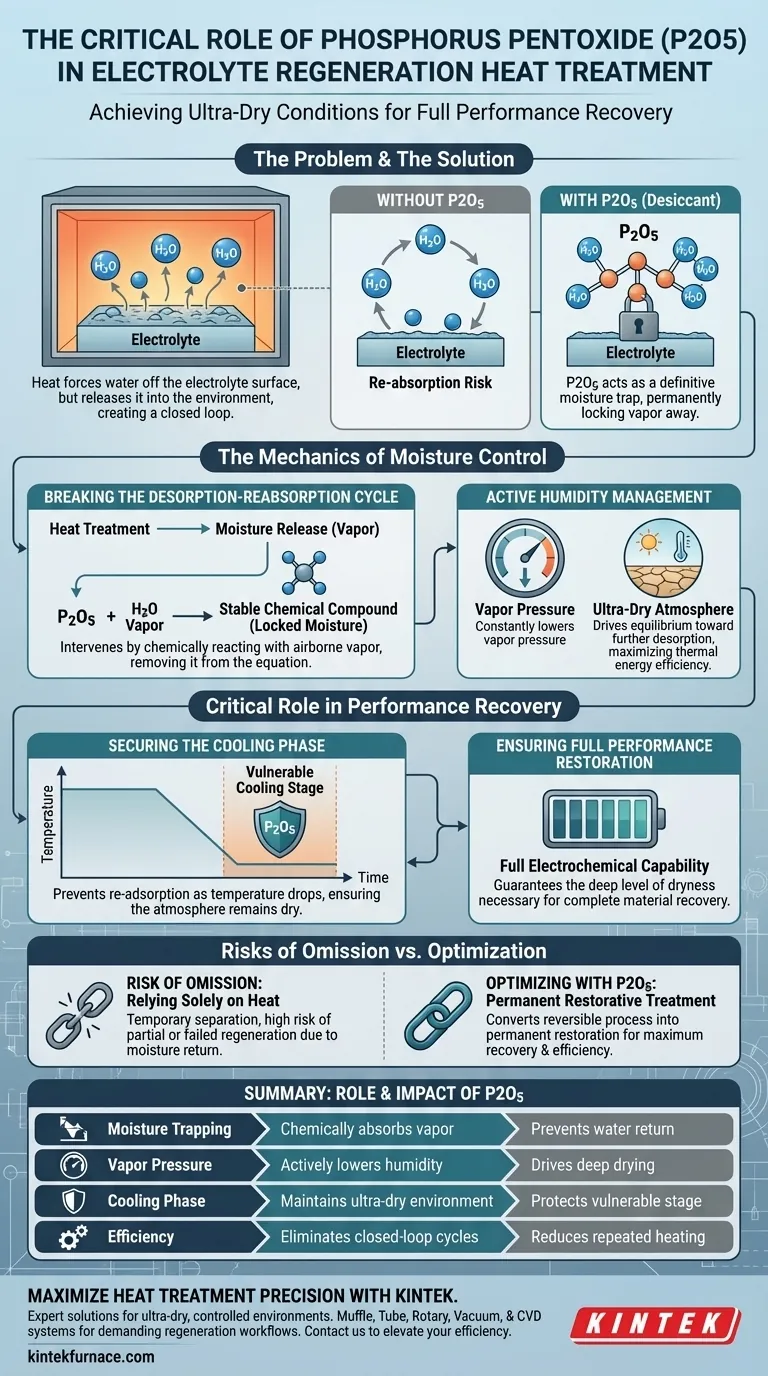
The primary purpose of adding phosphorus pentoxide (P2O5) during electrolyte regeneration is to act as a definitive moisture trap that prevents water from returning to the electrolyte. While heat forces water molecules off the electrolyte surface, P2O5 chemically absorbs this released vapor immediately. This ensures the moisture is permanently locked away, preventing it from re-adsorbing onto the material as the system cools.
While thermal treatment releases moisture from the electrolyte, it does not inherently remove it from the surrounding environment. Phosphorus pentoxide bridges this gap by chemically trapping the desorbed water, maintaining the ultra-dry atmosphere required to fully restore electrochemical performance.

The Mechanics of Moisture Control
Breaking the Desorption-Reabsorption Cycle
Heating an electrolyte is effective at breaking the bond between the material and adsorbed water molecules. However, this process simply releases the water into the immediate atmosphere of the reaction vessel.
Without a scavenging agent, this creates a closed loop where the moisture remains available in the environment. Phosphorus pentoxide intervenes in this cycle by reacting with the airborne water vapor, effectively removing it from the equation before it can interact with the electrolyte again.
Active Humidity Management
The regeneration process relies on maintaining an environment that is significantly drier than the electrolyte itself. P2O5 provides active management of environmental humidity throughout the treatment.
By constantly lowering the vapor pressure in the atmosphere, it drives the equilibrium toward further desorption. This ensures that the heat treatment occurs in an ultra-dry atmosphere, maximizing the efficiency of the thermal energy applied.
The Critical Role in Performance Recovery
Securing the Cooling Phase
The most vulnerable stage of regeneration is the cooling phase. As the temperature drops, the electrolyte becomes thermodynamically prone to re-absorbing moisture from its surroundings.
Because P2O5 has already locked the moisture away, the atmosphere remains dry even as the temperature decreases. This protection is vital for ensuring the electrolyte retains its regenerated state.
Ensuring Full Performance Restoration
The ultimate goal of this process is the recovery of the electrolyte's specific electrochemical capabilities.
Mere drying is often insufficient; the material requires deep regeneration to function correctly. The presence of P2O5 guarantees the level of dryness necessary for the full recovery of electrolyte performance.
Understanding the Risks of Omission
The Limits of Thermal Treatment
It is a common pitfall to assume that high temperatures alone are sufficient for regeneration.
Relying solely on heat creates a temporary separation of water and electrolyte. Without a desiccant like P2O5, you risk a partial or failed regeneration, as the moisture is likely to return to the surface once the heat source is removed.
Optimizing the Regeneration Process
If your primary focus is maximum performance recovery:
- Prioritize the inclusion of P2O5 to ensure an ultra-dry environment that prevents re-adsorption during the critical cooling phase.
If your primary focus is process efficiency:
- Recognize that adding P2O5 reduces the need for repeated heating cycles by permanently capturing moisture in a single pass.
By integrating phosphorus pentoxide, you convert a reversible thermal process into a permanent restorative treatment.
Summary Table:
| Feature | Role of P2O5 in Regeneration | Impact on Electrolyte Performance |
|---|---|---|
| Moisture Trapping | Chemically absorbs desorbed water vapor | Prevents water from returning to the material |
| Vapor Pressure | Actively lowers atmospheric humidity | Drives moisture equilibrium toward deep drying |
| Cooling Phase | Maintains ultra-dry environment | Protects the electrolyte during its most vulnerable stage |
| Efficiency | Eliminates closed-loop moisture cycles | Reduces the need for repeated thermal cycles |
Maximize Your Lab’s Heat Treatment Precision with KINTEK
Don't let moisture re-adsorption compromise your research results. Backed by expert R&D and world-class manufacturing, KINTEK provides high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed for the most demanding electrolyte regeneration workflows. Whether you require standard setups or fully customizable high-temp furnaces, our solutions ensure the ultra-dry, controlled environments your materials need for full performance restoration.
Ready to elevate your thermal processing efficiency? Contact KINTEK today to discuss your unique needs with our technical specialists.
Visual Guide
References
- Boyeong Jang, Yoon Seok Jung. Revitalizing Sulfide Solid Electrolytes for All‐Solid‐State Batteries: Dry‐Air Exposure and Microwave‐Driven Regeneration. DOI: 10.1002/aenm.202502981
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 304 316 Stainless Steel High Vacuum Ball Stop Valve for Vacuum Systems
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- How does the 1600°C range influence biomass microstructure? Transform Carbon into High-Performance Graphite
- Why must the entire system be maintained at a high temperature during the filling process of a sodium heat pipe?
- What function does a fluidized bed reactor perform in oil sludge pyrolysis? Enhance Thermal Efficiency
- How does oxygen-enhanced alkaline thermal treatment benefit high-purity cellulose pulp? Achieve Superior Fiber Yield
- How does increasing the soaking zone temperature in a walking-beam furnace affect Titanium/Steel clad plates?
- What are the primary advantages of using a downdraft fixed-bed reactor for co-gasification? Pure Syngas Made Simple
- What are the methods of heat transfer in furnaces? Master Heat Control for Better Results
- Why must catalysts undergo high-temperature pretreatment? Ensure Precise CO Oxidation Data with KINTEK



















