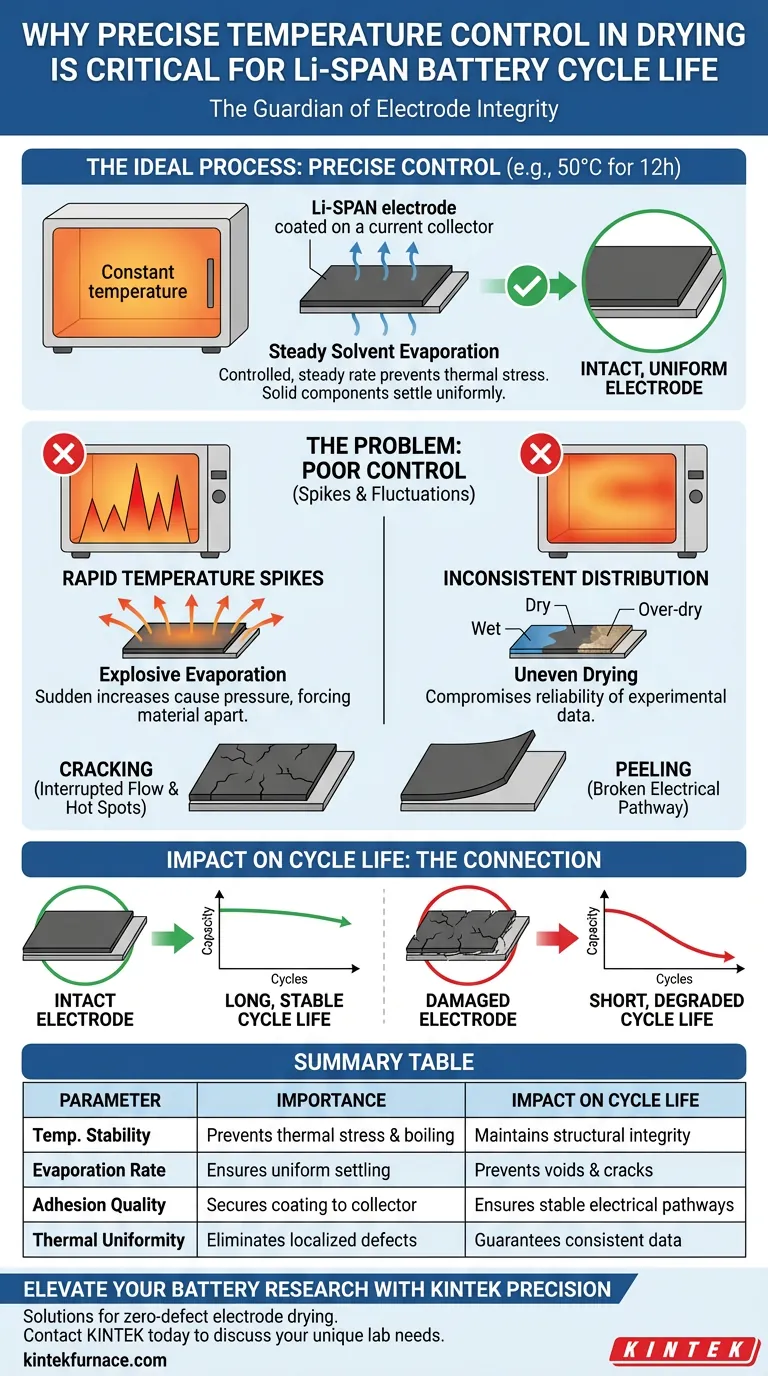
Precise temperature control is the guardian of electrode integrity. In the fabrication of Lithium-Sulfurized Polyacrylonitrile (Li-SPAN) batteries, maintaining a strict thermal environment—typically 50°C for 12 hours—is critical to ensure solvents evaporate at a controlled, steady rate. This regulation prevents the electrode coating from experiencing thermal stress, which otherwise leads to cracking or detachment from the current collector, thereby destroying the battery's potential for a long cycle life.
The stability of an electrode's physical structure defines its electrochemical longevity. Without precise temperature control during drying, mechanical failures like peeling inevitably lead to inconsistent performance and shortened cycle life.

The Physics of Electrode Drying
Regulating Solvent Evaporation
The primary goal of the constant temperature drying oven is to manage the removal of solvents used during the mixing process.
When temperature is controlled precisely, the solvent evaporates steadily. This gradual removal allows the solid components of the electrode to settle uniformly without creating internal voids or stress points.
Preventing Rapid Temperature Spikes
Sudden increases in temperature are detrimental to the electrode's microstructure.
If the oven fluctuates or heats too rapidly, the solvent may boil or evaporate explosively. This rapid expansion creates pressure that forces the material apart, damaging the delicate coating before it has fully set.
Connecting Structure to Cycle Life
Ensuring Adhesion to the Current Collector
For a battery to function, the active material must stay in physical contact with the current collector.
Precise temperature regulation ensures the coating dries without peeling off. If the coating detaches, the electrical pathway is broken, rendering that portion of the active material useless and significantly reducing the battery's capacity and lifespan.
Maintaining Surface Continuity
A uniform, unblemished surface is required for stable electrochemical reactions.
Controlled drying prevents cracking across the electrode surface. Cracks interrupt the flow of ions and electrons, leading to localized "hot spots" or dead zones that degrade the battery faster during repeated charging and discharging cycles.
Common Pitfalls and Trade-offs
The Risk of Rushing the Process
It is often tempting to increase temperatures to speed up the drying time.
However, deviating from the standard parameters (such as 50°C) risks introducing thermal shock. While the sample may appear dry faster, the microscopic structural damage incurred will result in immediate failure or rapid degradation during cycle testing.
Inconsistent Thermal Distribution
If the oven fails to maintain a constant temperature throughout the chamber, drying becomes uneven.
One part of the electrode may over-dry and crack while another remains solvent-heavy. This inconsistency compromises the reliability of experimental data, making it impossible to accurately judge the true performance of the Li-SPAN material.
Ensuring Long-Term Performance
To maximize the cycle life of your Li-SPAN batteries, the drying phase must be treated with the same precision as the chemical synthesis.
- If your primary focus is Structural Integrity: Adhere strictly to the 50°C protocol for the full 12 hours to guarantee zero cracking or peeling.
- If your primary focus is Reproducibility: Ensure your oven is calibrated to prevent fluctuations, securing the stability of electrochemical performance across multiple samples.
By prioritizing steady evaporation over speed, you secure the physical foundation required for superior electrochemical performance.
Summary Table:
| Parameter | Importance in Li-SPAN Fabrication | Impact on Battery Cycle Life |
|---|---|---|
| Temperature Stability | Prevents thermal stress and solvent boiling | Maintains electrode structural integrity |
| Evaporation Rate | Ensures uniform settling of solid components | Prevents internal voids and surface cracks |
| Adhesion Quality | Secures coating to the current collector | Ensures stable electrical pathways |
| Thermal Uniformity | Eliminates localized over-drying or wet spots | Guarantees consistent electrochemical data |
Elevate Your Battery Research with KINTEK Precision
Don’t let inconsistent thermal processing compromise your Li-SPAN electrode integrity. KINTEK provides industry-leading laboratory high-temperature solutions, including specialized drying ovens, muffle furnaces, and vacuum systems designed for the rigorous demands of battery R&D.
Backed by expert manufacturing, our equipment ensures the precise temperature regulation required to eliminate electrode cracking and peeling, securing the physical foundation for superior electrochemical performance. Whether you need standard or fully customizable heating systems, our experts are ready to help you optimize your fabrication process.
Ready to achieve zero-defect electrode drying?
Contact KINTEK today to discuss your unique lab needs
Visual Guide
References
- Krishna Kumar Sarode, Vibha Kalra. Solid–liquid–solid mediated artificial SEI coated stable lithium and high-sulfur percentage SPAN for high performance Li–S batteries. DOI: 10.1039/d3ya00423f
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- What is the purpose of using a laboratory drying oven for catalyst recycling? Optimize Performance & Data Integrity
- How does the temperature field provided by a High-Temperature Reaction Furnace promote pore development? 700-800°C Mastery
- Why is a high-temperature sintering furnace critical for BCZT ceramics? Achieving High Densification and Performance
- What protective roles does argon gas play in SiC sintering? Essential Insights for High-Purity Ceramics
- What is the importance of a stable thermal environment during crystallization? Ensure Precision in Metal Oxide Films
- Why is it necessary to dry Industrial EAF slag before hydrogen reduction? Crucial Safety and Accuracy Prep
- What is the function of a high-pressure hydrothermal reactor in hydrochar synthesis? Unlock Biomass Transformation
- Why is a high-pressure digestion tank essential for ZnO/rGO hydrothermal synthesis? Achieve superior interfacial coupling



















