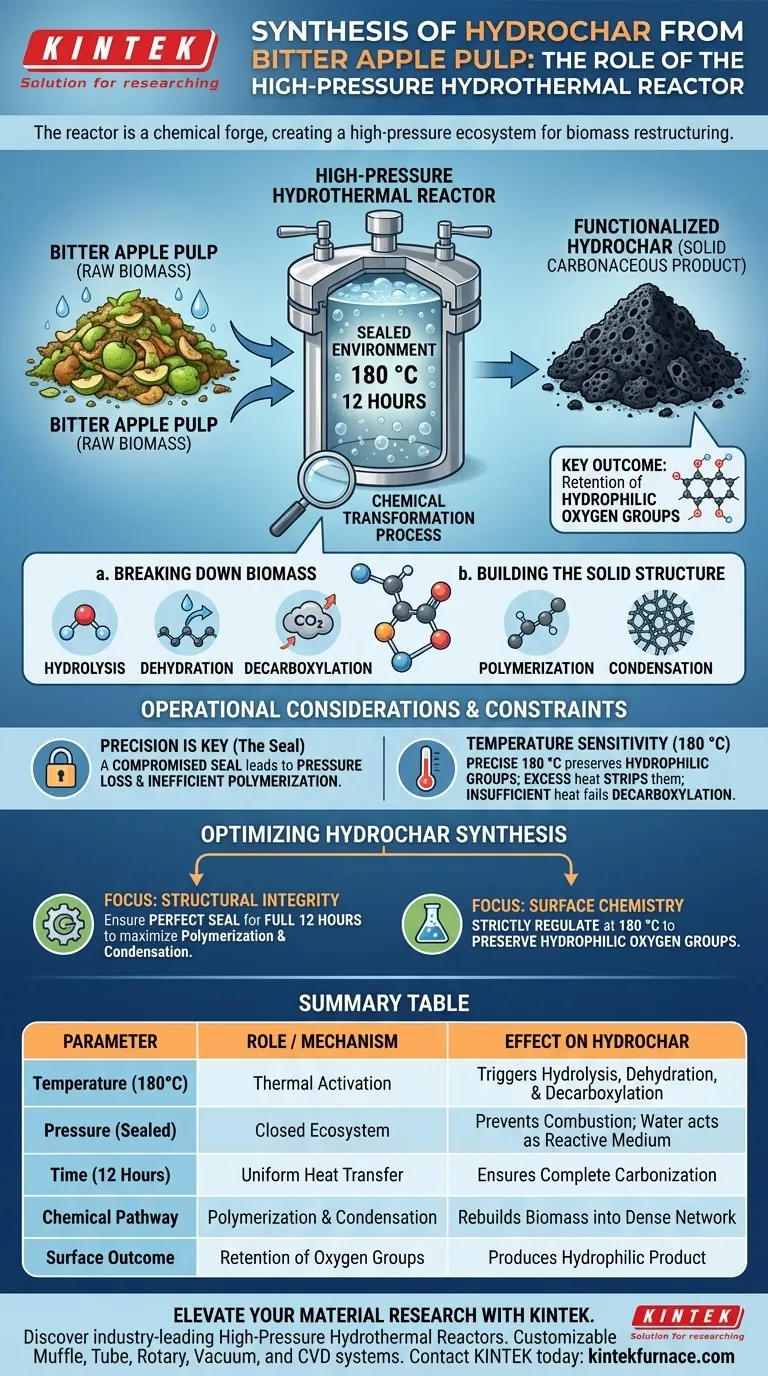
The high-pressure hydrothermal reactor serves as the critical transformation vessel in the synthesis of hydrochar from Bitter Apple Pulp. By maintaining a sealed environment at 180 °C for 12 hours, it generates the necessary pressure and thermal energy to force the physical and chemical conversion of raw biomass into a solid carbonaceous product.
Core Insight: The reactor is not merely a heating element; it creates a closed, high-pressure ecosystem that induces simultaneous breakdown and restructuring of biomass. This specific environment allows for the retention of hydrophilic oxygen groups, resulting in a chemically functionalized hydrochar rather than simple burnt carbon.

Creating the Conditions for Carbonization
The primary role of the reactor is to bridge the gap between raw organic pulp and stable carbon materials. It achieves this by strictly controlling two physical parameters: confinement and thermal duration.
The Role of the Sealed Environment
The reactor must be completely sealed to function correctly. This containment allows pressure to build up naturally as the temperature rises.
This high-pressure physical environment precludes the biomass from simply drying out or combusting as it would in an open fire. Instead, it forces water within the pulp to act as a reactive medium.
Operational Parameters
For Bitter Apple Pulp specifically, the reactor maintains a steady temperature of 180 °C.
This temperature is sustained for a duration of 12 hours. This extended timeframe ensures that heat transfer is uniform throughout the biomass, preventing unreacted cores within the pulp.
The Chemical Transformation Process
Inside the reactor, the high-pressure environment triggers a cascade of five specific chemical reactions. These mechanisms convert the biological structure of the pulp into a stable chemical structure.
Breaking Down the Biomass
The process begins with hydrolysis, where water molecules break down the complex bonds of the pulp.
Simultaneously, dehydration removes water from the molecular structure, and decarboxylation removes carboxyl groups, releasing carbon dioxide. These steps effectively strip away unstable elements from the raw pulp.
Building the Solid Structure
Once the biomass is broken down, the reactor facilitates polymerization and condensation.
These reactions rebuild the remaining molecules into a dense, solid network. This "re-assembling" is what forms the final solid carbonaceous product, known as hydrochar.
Functionalizing the Surface
Unlike standard pyrolysis (heating without oxygen), this hydrothermal process preserves specific surface properties.
The resulting hydrochar possesses hydrophilic oxygen groups. These groups are essential for applications where the char needs to interact with water or other polar substances.
Operational Considerations and Constraints
While the reactor is a powerful tool, the quality of the output depends heavily on the integrity of the process conditions.
The Necessity of Precision
The distinction between creating high-quality hydrochar and creating inert sludge lies in the stability of the reactor.
If the seal is compromised, pressure is lost, and the polymerization and condensation steps may fail to occur efficiently.
Temperature Sensitivity
The specific set point of 180 °C is calibrated to induce carbonization without destroying the hydrophilic oxygen groups.
Excessive temperatures could strip these groups away, while insufficient heat would fail to trigger the necessary decarboxylation, leaving the pulp under-processed.
Optimizing Hydrochar Synthesis
To maximize the utility of the hydrochar produced from Bitter Apple Pulp, focus on controlling the reaction environment.
- If your primary focus is Structural Integrity: Ensure the reactor maintains a perfect seal for the full 12 hours to maximize polymerization and condensation.
- If your primary focus is Surface Chemistry: Strictly regulate the temperature at 180 °C to preserve the hydrophilic oxygen groups essential for chemical reactivity.
The high-pressure hydrothermal reactor effectively acts as a chemical forge, using pressure and time to restructure Bitter Apple Pulp into a functional, stable carbon material.
Summary Table:
| Parameter | Role / Mechanism | Effect on Hydrochar |
|---|---|---|
| Temperature (180°C) | Thermal Activation | Triggers hydrolysis, dehydration, and decarboxylation |
| Pressure (Sealed) | Closed Ecosystem | Prevents combustion; forces water to act as a reactive medium |
| Time (12 Hours) | Uniform Heat Transfer | Ensures complete carbonization and prevents unreacted cores |
| Chemical Pathway | Polymerization & Condensation | Rebuilds biomass into a dense, solid carbonaceous network |
| Surface Outcome | Retention of Oxygen Groups | Produces a chemically functionalized, hydrophilic product |
Elevate Your Material Research with KINTEK
Ready to transform biomass into high-value carbon materials? KINTEK provides industry-leading high-pressure hydrothermal reactors designed for precision and durability. Backed by expert R&D and manufacturing, we offer customizable Muffle, Tube, Rotary, Vacuum, and CVD systems, specifically engineered for laboratories and industrial synthesis.
Whether you are processing Bitter Apple Pulp or advanced polymers, our high-temp furnaces ensure the stable environment required for successful polymerization and surface functionalization. Contact KINTEK today to find the perfect reactor for your unique needs!
Visual Guide
References
- Himanshu Gupta, Debasish Sarkar. Bitter Apple Pulp‐Derived Porous Carbon with Rich Oxygen Functionalities for High‐Performance Zinc‐Ion Storage. DOI: 10.1002/smll.202502071
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- Why is a constant temperature drying oven utilized at 40 °C for clayey raw materials? Ensure Mineral Integrity.
- What are some common applications of PVD? Boost Performance with Advanced Thin-Film Coatings
- What is the synergistic effect of industrial fly ash in beet pulp porous carbons? Enhance 3D Structural Performance
- What is the core role of a high-pressure autoclave in the synthesis of LTA zeolites? Achieve Precise Crystal Growth
- How does the SCRS model simplify furnace combustion simulation? Efficiency Meets Accuracy in Thermal Modeling
- Why is a laboratory resistance furnace preferred for Al-5Er-Ti alloys? Achieve High-Purity Homogeneity
- What role does a PID controller play in the calcination process of eggshells? Precision Control for Pure Calcium Oxide
- What are the advantages of solution combustion synthesis? Optimize (MnFeNiCoX)3O4 High-Entropy Oxide Catalyst Production



















