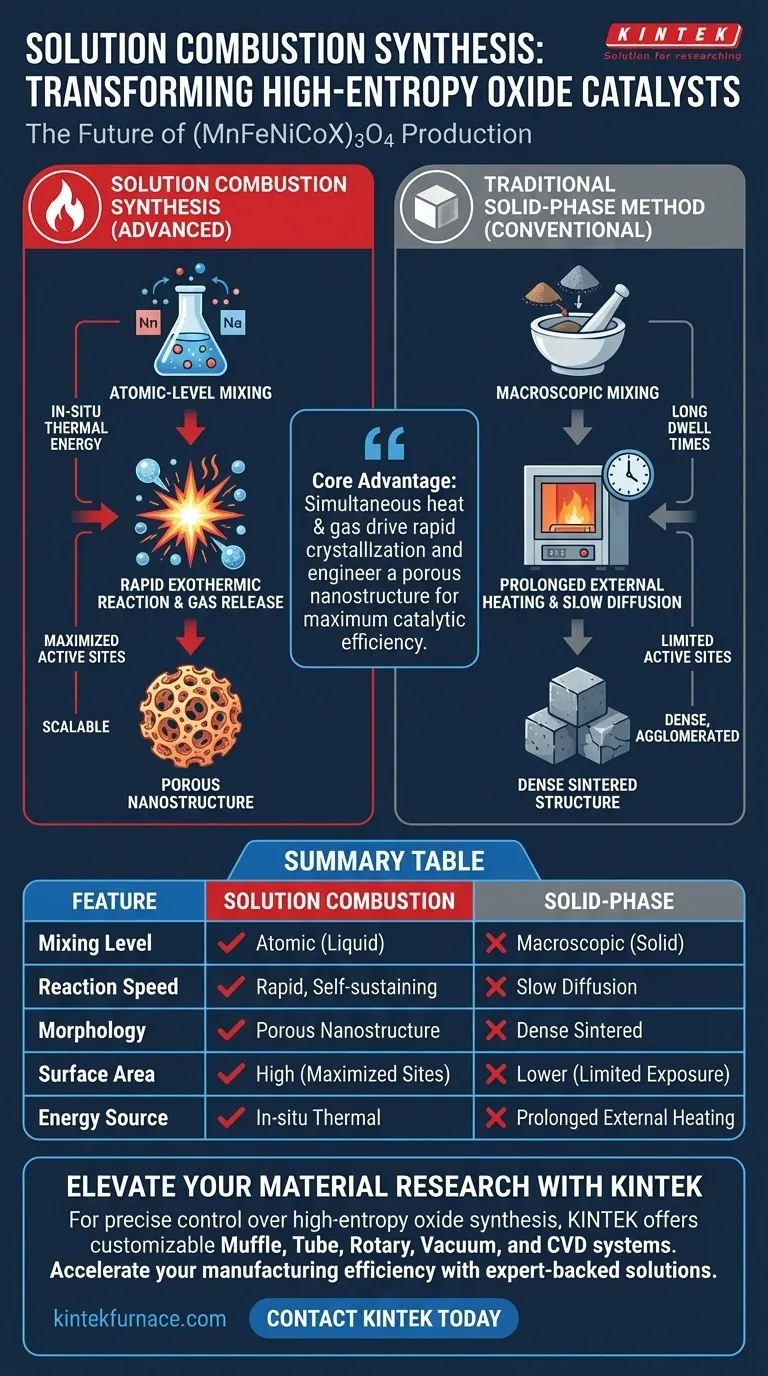
Solution combustion synthesis provides a distinct advantage in both manufacturing efficiency and material performance compared to traditional solid-phase methods. By utilizing an atomic-level mixture of metal nitrates and fuels to trigger a rapid exothermic reaction, this technique overcomes the diffusion limitations of solid-state processing to produce high-entropy oxides with superior structural properties.
The core advantage of this method is the simultaneous generation of heat and gas. This dual mechanism drives the rapid crystallization of the spinel phase while naturally engineering a porous nanostructure that maximizes catalytic efficiency.
Transforming Production Efficiency
Rapid Reaction Kinetics
Traditional solid-phase methods often rely on slow heating and long dwell times to induce phase changes. In contrast, solution combustion synthesis utilizes a rapid, exothermic redox reaction.
This reaction generates significant in-situ thermal energy. This internal heat source is sufficient to drive the immediate formation of the complex (MnFeNiCoX)3O4 spinel phase without the need for prolonged external heating.
Scalability and Throughput
The efficiency of the combustion process translates directly to scalability. Because the reaction is fast and self-sustaining once initiated, it offers higher production efficiency than solid-phase techniques.
This makes the method particularly attractive for moving from laboratory-scale synthesis to larger manufacturing volumes.
Optimizing Catalyst Morphology
Atomic-Level Homogeneity
High-entropy oxides require the uniform distribution of multiple elements. Solution combustion synthesis begins by mixing metal nitrate oxidizers and fuels (such as urea) at the atomic level.
This ensures that the constituent elements are perfectly blended before the reaction starts, leading to a consistent chemical composition in the final product.
Engineering Porosity via Gas Evolution
A unique byproduct of the combustion reaction is the release of large volumes of gas. As the material forms, this gas escape creates a "leavening" effect.
The result is a loose, porous nanostructure. Unlike solid-phase methods, which can lead to dense, sintered blocks, combustion synthesis naturally inhibits agglomeration.
Maximizing Active Sites
The physical structure of a catalyst dictates its performance. The porous architecture created by gas evolution significantly increases the specific surface area of the material.
This structural openness enhances the exposure of catalytic active sites. More exposed sites mean better interaction with reactants, directly improving the catalytic performance of the high-entropy oxide.
Understanding the Process Dynamics
Reliance on Specific Precursors
The process is chemically specific, requiring metal nitrates as oxidizers and specific fuels like urea.
This requirement dictates the supply chain, as you cannot simply substitute these with the oxides or carbonates often used in solid-state synthesis.
Managing Exothermic Intensity
The reaction is described as rapid and exothermic. While this provides the energy for phase formation, the intensity of this heat release is a critical variable.
Control over the fuel-to-oxidizer ratio is essential to manage this energy release and ensure the desired porous morphology is achieved without excessive sintering.
Strategic Application for Catalyst Development
To determine if solution combustion synthesis is the right approach for your (MnFeNiCoX)3O4 project, consider your primary constraints:
- If your primary focus is catalytic performance: This method is superior because it naturally generates the porous nanostructure required to maximize active site exposure.
- If your primary focus is manufacturing speed: The rapid, self-sustaining nature of the reaction offers higher production efficiency and scalability compared to slow solid-phase diffusion.
Solution combustion synthesis effectively couples the need for high-throughput manufacturing with the requirement for intricate, high-surface-area material design.
Summary Table:
| Feature | Solution Combustion Synthesis | Traditional Solid-Phase Method |
|---|---|---|
| Mixing Level | Atomic-level homogeneity (liquid) | Macroscopic mixing (solid) |
| Reaction Speed | Rapid, self-sustaining exothermic reaction | Slow diffusion, long dwell times |
| Morphology | Loose, porous nanostructure | Dense, often sintered/agglomerated |
| Surface Area | High (maximized active sites) | Lower (limited exposure) |
| Energy Source | In-situ thermal energy from redox | Prolonged external heating |
Elevate Your Material Research with KINTEK
Precise control over high-entropy oxide synthesis requires equipment that can handle demanding thermal profiles and specific gas environments. KINTEK provides industry-leading R&D and manufacturing solutions, offering customizable Muffle, Tube, Rotary, Vacuum, and CVD systems designed to support innovative processes like solution combustion synthesis.
Whether you are scaling up (MnFeNiCoX)3O4 production or engineering the next generation of porous catalysts, our expert-backed high-temperature furnaces deliver the uniformity and reliability your lab needs. Contact KINTEK today to discuss your unique furnace requirements and see how our tailored high-temp solutions can accelerate your manufacturing efficiency.
Visual Guide
References
- Milad Zehtab Salmasi, Hua Song. Tuning High-Entropy Oxides for Oxygen Evolution Reaction Through Electrocatalytic Water Splitting: Effects of (MnFeNiCoX)3O4 (X = Cr, Cu, Zn, and Cd) on Electrocatalytic Performance. DOI: 10.3390/catal15090827
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Magnesium Extraction and Purification Condensing Tube Furnace
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What role does a high-temperature heating environment play in the hydrothermal synthesis of ZSM-5 zeolite crystals?
- Why is precise temperature control critical for drying carbon nanotube films? Achieve Perfect 80°C Thermal Stability
- How do heat treatment furnaces function? Master Thermal Control and Atmosphere for Superior Material Properties
- What is the purpose of using a laboratory blast drying oven at 107°C for 17 hours for reforming catalysts?
- How does a high-temperature annealing furnace regulate cold-rolled steel? Optimize Manganese Steel Performance
- What is the purpose of high-vacuum thermal evaporation coating equipment in SiQD LED fabrication? Expert Insights
- Why must high-purity nitrogen be used for biochar activation? Ensure Carbon Integrity and Pore Development
- Why is an environmental laboratory chamber equipped with an optical window required for synthesizing Hafnium Carbide?



















