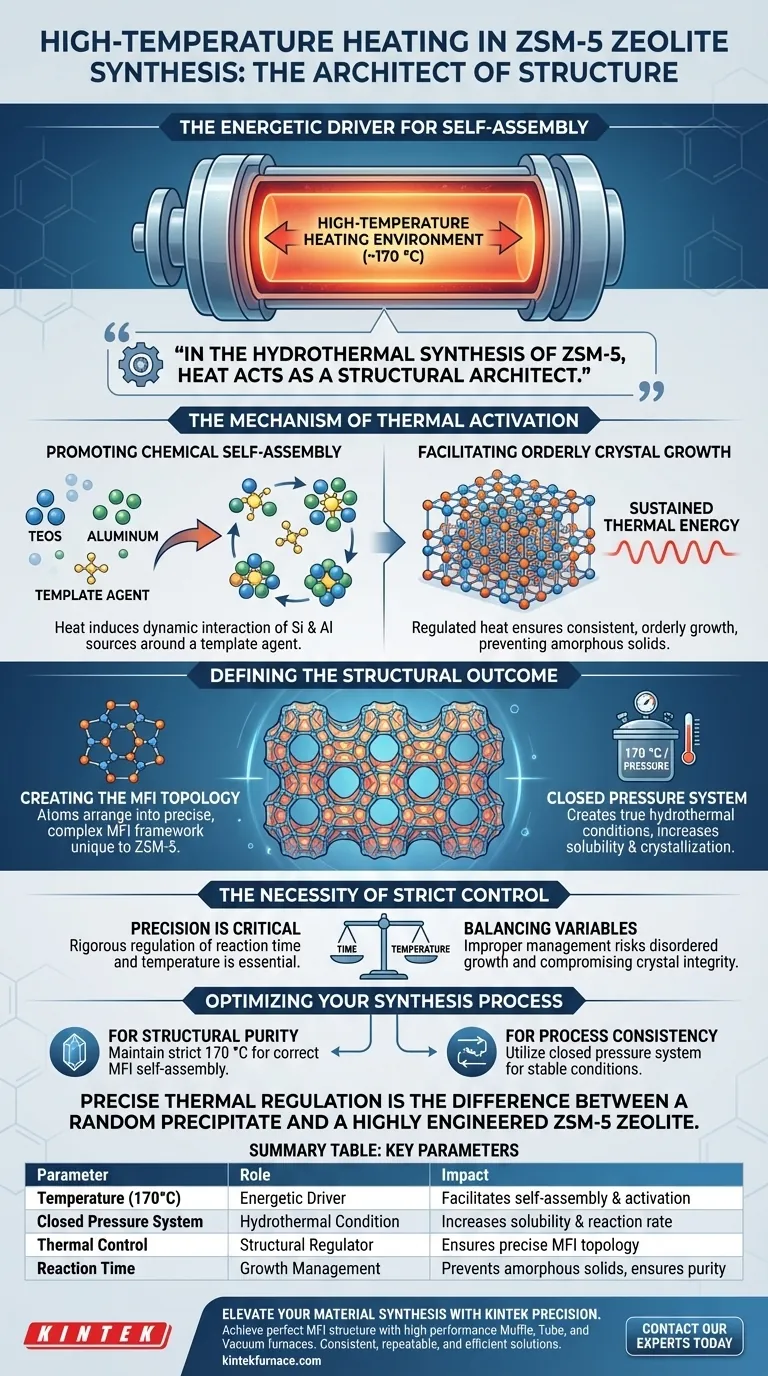
A high-temperature heating environment serves as the energetic driver for the self-assembly of ZSM-5 zeolite crystals. specifically, maintaining a temperature of approximately 170 °C within a closed pressure system forces the silicon (e.g., TEOS) and aluminum sources to organize around a template agent. This thermal input is not merely about speed; it is fundamental to creating the specific MFI topological structure required for the zeolite's function.
In the hydrothermal synthesis of ZSM-5, heat acts as a structural architect. It generates the necessary pressure and activation energy to transform raw silicon and aluminum sources into a highly ordered, crystalline MFI framework.

The Mechanism of Thermal Activation
Promoting Chemical Self-Assembly
The primary function of the high-temperature environment is to induce self-assembly.
At ambient temperatures, silicon sources (such as TEOS) and aluminum sources remain relatively inert regarding crystal formation. The introduction of heat encourages these components to interact dynamically in the presence of a template agent.
Facilitating Orderly Crystal Growth
The 170 °C environment ensures that the interaction between precursors is regulated and consistent.
By sustaining this specific thermal energy, the system promotes the orderly growth of the crystal lattice. This prevents the formation of amorphous solids and ensures the development of the distinct crystalline structure defining ZSM-5.
Defining the Structural Outcome
Creating the MFI Topology
The ultimate goal of this thermal process is the formation of a specific MFI topological structure.
This complex framework is unique to ZSM-5 zeolites. The high-temperature environment provides the thermodynamic conditions necessary for the atoms to arrange themselves into this precise geometry.
The Role of the Closed System
The reference highlights that this heating occurs within a closed pressure system.
Heating the synthesis mixture to 170 °C inside a sealed vessel creates true hydrothermal conditions. The resulting pressure, combined with the heat, increases the solubility of the reactants and facilitates the crystallization process.
The Necessity of Strict Control
Precision is Critical
While high temperature is the engine of synthesis, strict control is the steering wheel.
The reference emphasizes that both reaction time and temperature must be regulated rigorously. A synthesis environment that fluctuates significantly from 170 °C risks compromising the integrity of the crystal structure.
Balancing Time and Temperature
The relationship between the heating duration and the temperature intensity determines the final quality of the zeolite.
Improper management of these variables can lead to disordered growth or failure to achieve the desired MFI structure. The system relies on a consistent environment to ensure every crystal forms correctly.
Optimizing Your Synthesis Process
To ensure the successful production of high-quality ZSM-5 crystals, focus on the following control parameters:
- If your primary focus is Structural Purity: Maintain the temperature strictly at 170 °C to guarantee the correct self-assembly of the MFI topology.
- If your primary focus is Process Consistency: Utilize a closed pressure system to ensure stable hydrothermal conditions throughout the reaction time.
Precise thermal regulation is the difference between a random precipitate and a highly engineered ZSM-5 zeolite.
Summary Table:
| Parameter | Role in ZSM-5 Synthesis | Impact on Outcome |
|---|---|---|
| Temperature (170°C) | Energetic Driver / Architect | Facilitates self-assembly and activation energy |
| Closed Pressure System | Hydrothermal Condition | Increases precursor solubility and reaction rate |
| Thermal Control | Structural Regulator | Ensures formation of precise MFI topology |
| Reaction Time | Growth Management | Prevents amorphous solids; ensures crystal purity |
Elevate Your Material Synthesis with KINTEK Precision
Achieving the perfect MFI topological structure in ZSM-5 synthesis requires uncompromising thermal accuracy. Backed by expert R&D and world-class manufacturing, KINTEK provides high-performance Muffle, Tube, and Vacuum furnace systems specifically designed to maintain the strict hydrothermal conditions your research demands. Whether you need a standard setup or a fully customizable solution for unique laboratory needs, our equipment ensures your process is consistent, repeatable, and efficient.
Ready to optimize your zeolite production? Contact our experts today to find your ideal high-temperature solution!
Visual Guide
References
- Wei Xiong, Jun Zhao. Acidic Site-Controlled ZSM-5 Catalysts for Fast Molten-Phase Pyrolysis of Plastic Waste with Tunable Product Distribution. DOI: 10.1021/acs.energyfuels.5c02781
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the role of a high-energy ball mill in NiWO4/GO preparation? Master High-Performance Composite Synthesis
- What is the purpose of using a rotary evaporator or a vacuum drying oven? Preserving SiC Powder Quality Post-Milling
- How does an annealing furnace work? A Guide to Controlled Heat Treatment
- What is the function of a laboratory vacuum drying oven in alpha-K3[CuIO6]·4H2O synthesis? Protect Crystal Integrity
- What is the role of industrial electric drying ovens in FDSSC titanium photoanode treatment? Enhance Solar Efficiency
- Why is a Cold Isostatic Press (CIP) utilized for LLTO samples? Achieve 98% Relative Density in Ceramics
- What is the purpose of introducing a pure iron interlayer between the titanium layer and the steel layer? Enhancing Bond Integrity
- What is the purpose of using a laboratory electric thermostatic blast drying oven in the pretreatment of sludge? Efficiency & Accuracy



















