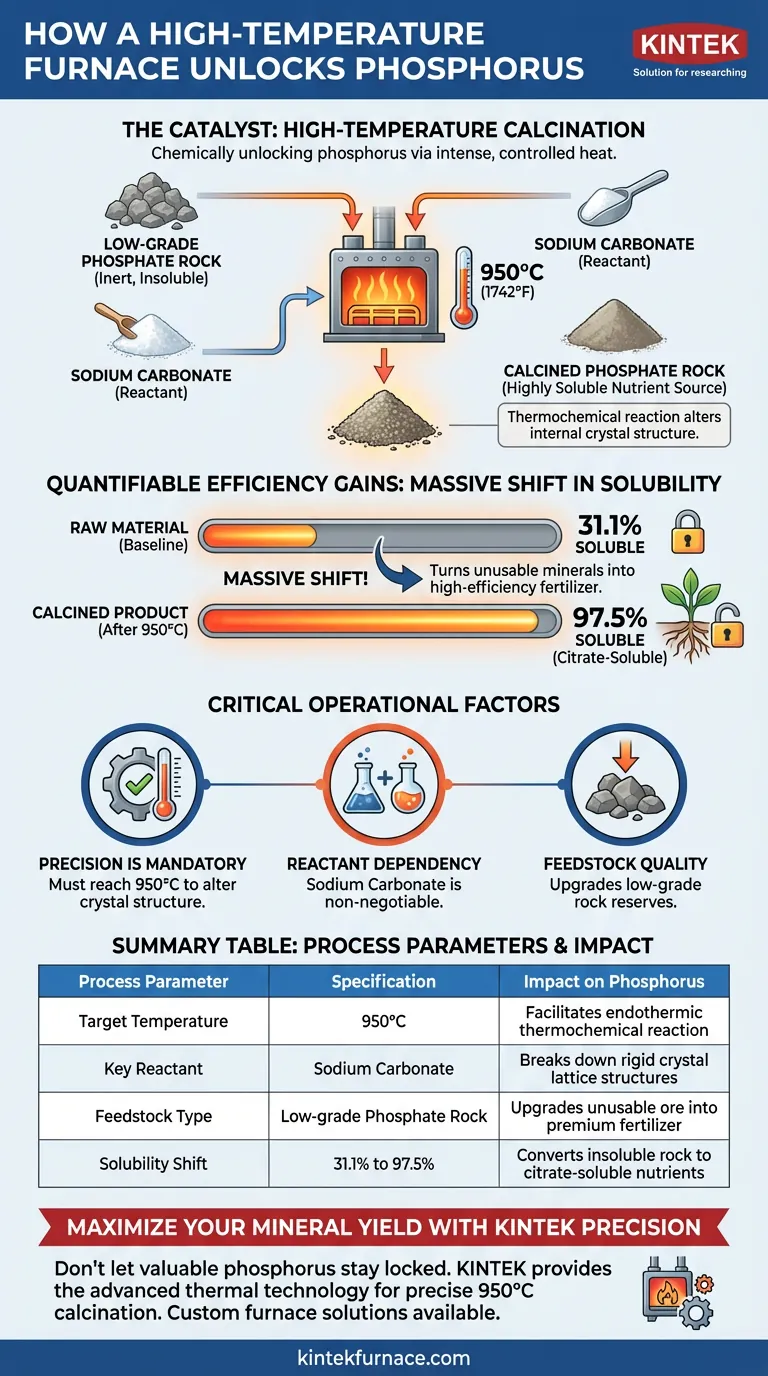
High-temperature calcination is the catalyst for chemically unlocking phosphorus. It enhances availability by driving a thermochemical reaction between low-grade phosphate rock and sodium carbonate at temperatures reaching approximately 950°C. This intense, controlled heat fundamentally alters the rock’s internal crystal structure, converting inert, insoluble material into a highly soluble nutrient source for plants.
By applying controlled thermal energy to low-grade phosphate rock, producers can trigger a massive shift in solubility—potentially rising from 31.1% to 97.5%. This process effectively turns raw, unusable minerals into high-efficiency, citrate-soluble fertilizer.

The Mechanism of Transformation
To understand how availability is enhanced, one must look at the specific chemical interactions driven by the furnace.
The Role of Sodium Carbonate
The process is not simply about heating rock; it is a chemical reaction. The furnace facilitates the interaction between low-grade phosphate rock and sodium carbonate.
Achieving Critical Temperature
This reaction is endothermic and requires significant energy. The furnace must sustain temperatures of roughly 950°C to drive the process forward.
Controlled Thermal Energy
The energy provided is "controlled," meaning the furnace maintains a stable environment. This stability is required to ensure the reaction reaches completion throughout the entire batch of material.
Structural Changes and Solubility
The heat serves a specific purpose: changing the physical and chemical architecture of the material.
Altering the Crystal Lattice
At 950°C, the internal crystal structure of the phosphate rock is modified. The heat breaks down the rigid bonds that keep phosphorus locked in an insoluble state.
Creating Citrate-Soluble Phosphorus
The result of this structural change is the conversion of insoluble phosphates into citrate-soluble phosphorus. This specific form of phosphorus is readily absorbable by crop root systems.
Quantifiable Efficiency Gains
The impact on availability is drastic rather than incremental. Data indicates that solubility can improve from a baseline of 31.1% to 97.5%, making the final product comparable to high-efficiency chemical fertilizers.
Critical Operational Factors
While the process is powerful, it relies on strict adherence to specific operational parameters.
Precision is Mandatory
The shift in solubility is contingent on reaching the target temperature of 950°C. Falling short of this thermal threshold will likely fail to alter the crystal structure sufficiently, leaving the phosphorus insoluble.
Reactant Dependency
Heat alone is insufficient. The presence of sodium carbonate is a non-negotiable requirement for the thermochemical reaction to occur.
Feedstock Quality
This method is specifically highlighted for processing low-grade phosphate rock. It is a value-add process designed to upgrade inferior materials that cannot be used in their raw state.
Maximizing Production Value
The use of a high-temperature furnace is ultimately about resource efficiency and product quality.
- If your primary focus is maximizing nutrient availability: Ensure your furnace controls are calibrated to maintain 950°C consistently, as this directly correlates to achieving the 97.5% solubility target.
- If your primary focus is resource utilization: Leverage this method to process low-grade phosphate rock reserves, converting otherwise lower-value ore into premium fertilizer.
By mastering the thermochemical balance of heat and sodium carbonate, you transform inert stone into a vital agricultural resource.
Summary Table:
| Process Parameter | Specification | Impact on Phosphorus |
|---|---|---|
| Target Temperature | 950°C | Facilitates endothermic thermochemical reaction |
| Key Reactant | Sodium Carbonate | Breaks down rigid crystal lattice structures |
| Feedstock Type | Low-grade Phosphate Rock | Upgrades unusable ore into premium fertilizer |
| Solubility Shift | 31.1% to 97.5% | Converts insoluble rock to citrate-soluble nutrients |
Maximize Your Mineral Yield with KINTEK Precision
Don't let valuable phosphorus stay locked in low-grade ore. KINTEK provides the advanced thermal technology required to achieve the precise 950°C threshold necessary for successful calcination.
Backed by expert R&D and world-class manufacturing, we offer a full range of Muffle, Tube, Rotary, Vacuum, and CVD systems. Whether you are processing phosphate rock or developing novel chemical reactions, our lab high-temp furnaces are fully customizable to meet your unique production needs.
Ready to transform your material processing efficiency? Contact us today to speak with our technical specialists about a custom furnace solution.
Visual Guide
References
- Andressa Nakagawa, Papa Saliou Sarr. Calcined low-grade phosphate rock fertilization enhances nitrogen fixation, yield, and grain quality in soybeans. DOI: 10.3389/fpls.2025.1581961
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What is the objective of GC-MS analysis on bio-oil? Unlock Chemical Value and Industrial Utility
- What is the purpose of using an industrial oven for low-temperature drying? Expert Glass Processing Guide
- Why is MgO used as a hard template for waste PET to carbon conversion? Unlock 3D Porous Structures
- What is the significance of preheating UHPC molds? Ensure Safety & Longevity with High-Temp Furnaces
- What is preventive maintenance on a furnace? A Proactive Strategy for Peak Performance
- What is the role of a laboratory vacuum drying oven in LNMO electrode slurry preparation? Master Solvent Removal
- What role does activation treatment play in converting PPS waste? Unlock High-Performance Energy Storage Pores
- What are the primary purposes of using high-purity argon flow during the pyrolysis of CMS membranes? Achieve High-Purity Results



















