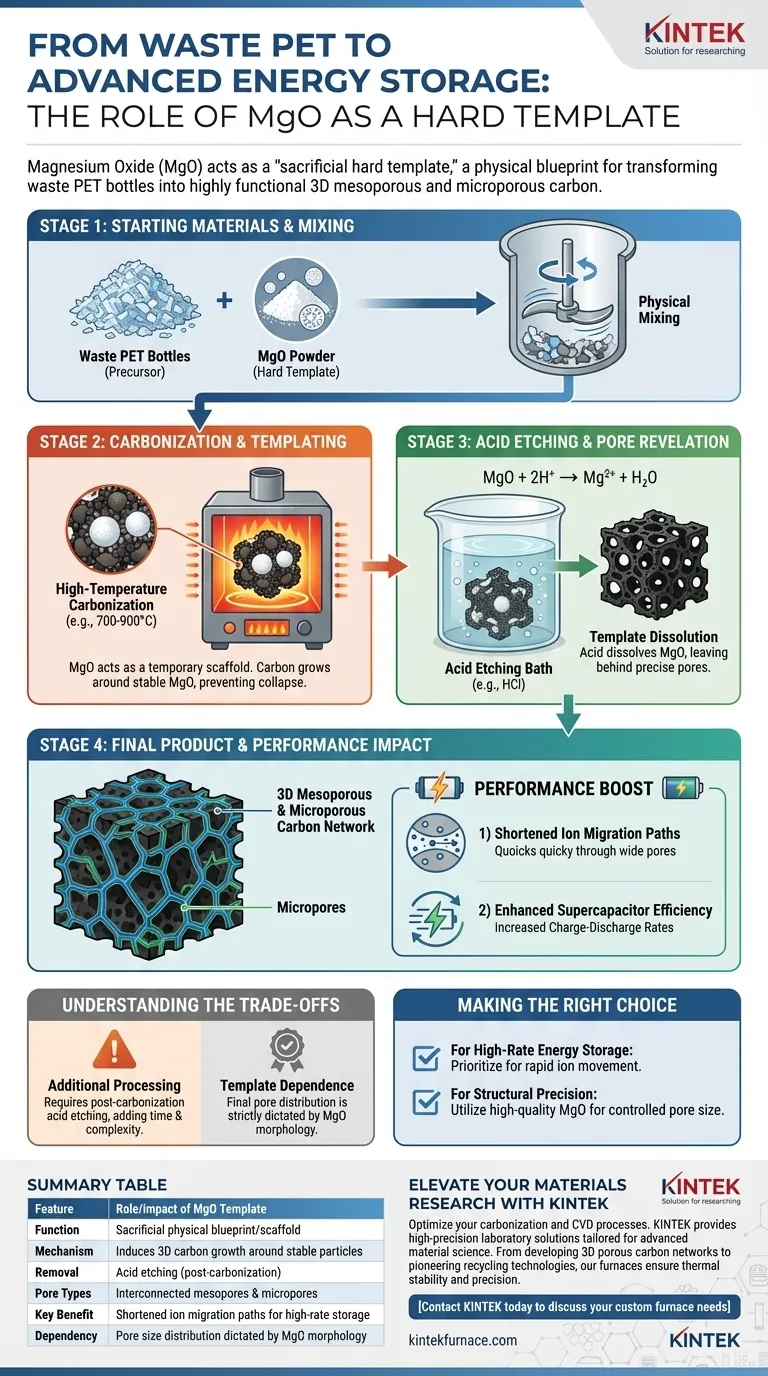
Magnesium Oxide (MgO) serves as a physical blueprint. It is introduced into the processing of waste PET bottles to act as a "sacrificial hard template" that dictates the internal architecture of the resulting carbon material. By occupying specific space during the high-temperature carbonization process, the MgO powder forces the carbon to grow around its particles, effectively molding the carbon into a specific shape before being chemically removed.
Core Takeaway MgO acts as a temporary scaffold that induces the formation of a highly interconnected 3D carbon network. Once the MgO is dissolved, it leaves behind a precise distribution of mesopores and micropores, which is critical for enhancing the performance of supercapacitors.

The Mechanism of Hard Templating
The Role of the "Sacrificial" Template
In this process, MgO is not intended to be part of the final product. It is mixed with the PET solely to shape the material during its transition from plastic to carbon.
Inducing the Carbon Skeleton
During the high-temperature furnace treatment, the PET decomposes and carbonizes. Because the MgO powder is thermally stable, it remains solid, forcing the forming carbon skeleton to develop around the MgO particles rather than collapsing into a dense block.
Creating the 3D Porous Network
The Etching Process
Once the carbonization is complete, the composite material undergoes acid etching. This chemical bath dissolves the MgO template completely, leaving the carbon structure intact.
Revealing the Pore Structure
Where the MgO particles once sat, voids remain. The removal of the template reveals a highly interconnected 3D network of mesopores and micropores. The size and distribution of these pores are directly controlled by the physical morphology of the MgO powder used at the start.
The Impact on Performance
Shortening Ion Migration Paths
The specific 3D structure created by the MgO template is not merely for texture; it serves a functional purpose. The interconnected pores significantly reduce the distance ions must travel within the material.
Enhancing Supercapacitor Efficiency
By facilitating faster ion movement, the templated carbon allows for rapid energy transfer. This directly translates to increased charge-discharge rates in supercapacitors, making the device more efficient and responsive.
Understanding the Trade-offs
Additional Processing Steps
While effective, using MgO as a hard template introduces complexity. It requires a post-carbonization acid etching step to remove the template, which adds time and chemical handling requirements to the manufacturing workflow compared to simple carbonization.
Dependence on Template Quality
The final properties of the carbon are strictly bound to the quality of the template. The pore size distribution is only as precise as the morphology of the MgO powder introduced; if the template is inconsistent, the final carbon network will be inconsistent.
Making the Right Choice for Your Goal
The use of MgO is a strategic engineering decision designed to maximize electrochemical performance.
- If your primary focus is High-Rate Energy Storage: Prioritize this method, as the shortened ion migration paths are essential for maximizing charge-discharge speeds.
- If your primary focus is Structural Precision: Utilize high-quality MgO powder, as its specific morphology directly dictates the control you have over the final pore size distribution.
By treating MgO as a temporary architect, you transform waste plastic into a highly tuned material optimized for rapid energy storage.
Summary Table:
| Feature | Role/Impact of MgO Template |
|---|---|
| Function | Sacrificial physical blueprint/scaffold |
| Mechanism | Induces 3D carbon growth around stable MgO particles |
| Removal Method | Acid etching (post-carbonization) |
| Pore Types | Interconnected mesopores and micropores |
| Key Benefit | Shortened ion migration paths for high-rate energy storage |
| Dependency | Pore size distribution is dictated by MgO morphology |
Elevate Your Materials Research with KINTEK
Are you looking to optimize your carbonization and chemical vapor deposition processes? KINTEK provides high-precision laboratory solutions tailored for advanced material science. Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique research requirements.
Whether you are developing 3D porous carbon networks or pioneering sustainable recycling technologies, our high-temperature furnaces ensure the thermal stability and precision your project demands. Contact KINTEK today to discuss your custom furnace needs and see how we can enhance your lab's efficiency and innovation.
Visual Guide
References
- Perseverance Dzikunu, Pedro Vilaça. Waste-to-carbon-based supercapacitors for renewable energy storage: progress and future perspectives. DOI: 10.1007/s40243-024-00285-4
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- What are the requirements for sulfur powder loading in MoS2 synthesis? Master the 50-150 mg Precision Range
- Why are high-precision nitrogen flow meters essential during pyrolysis? Ensure Perfect Char Preparation
- Why is a high-precision mass flow controller essential for iron ore reduction studies involving water vapor?
- What advantages does AlMe2iPrO (DMAI) offer over Trimethylaluminum (TMA)? Achieve Superior Area Selectivity
- Why is an electric heating furnace integrated with a capsule-piercing reactor? Ensure Precise Fluid Analysis
- What is the function of a high-temperature heating reactor in OPF delignification? Unlock High-Purity Cellulose
- What is the importance of providing technical documentation for high-temperature furnaces in multiple languages?
- How does a high-precision Vertical Bridgman Furnace facilitate ZnGeP2 growth? Master Single Crystal Production








