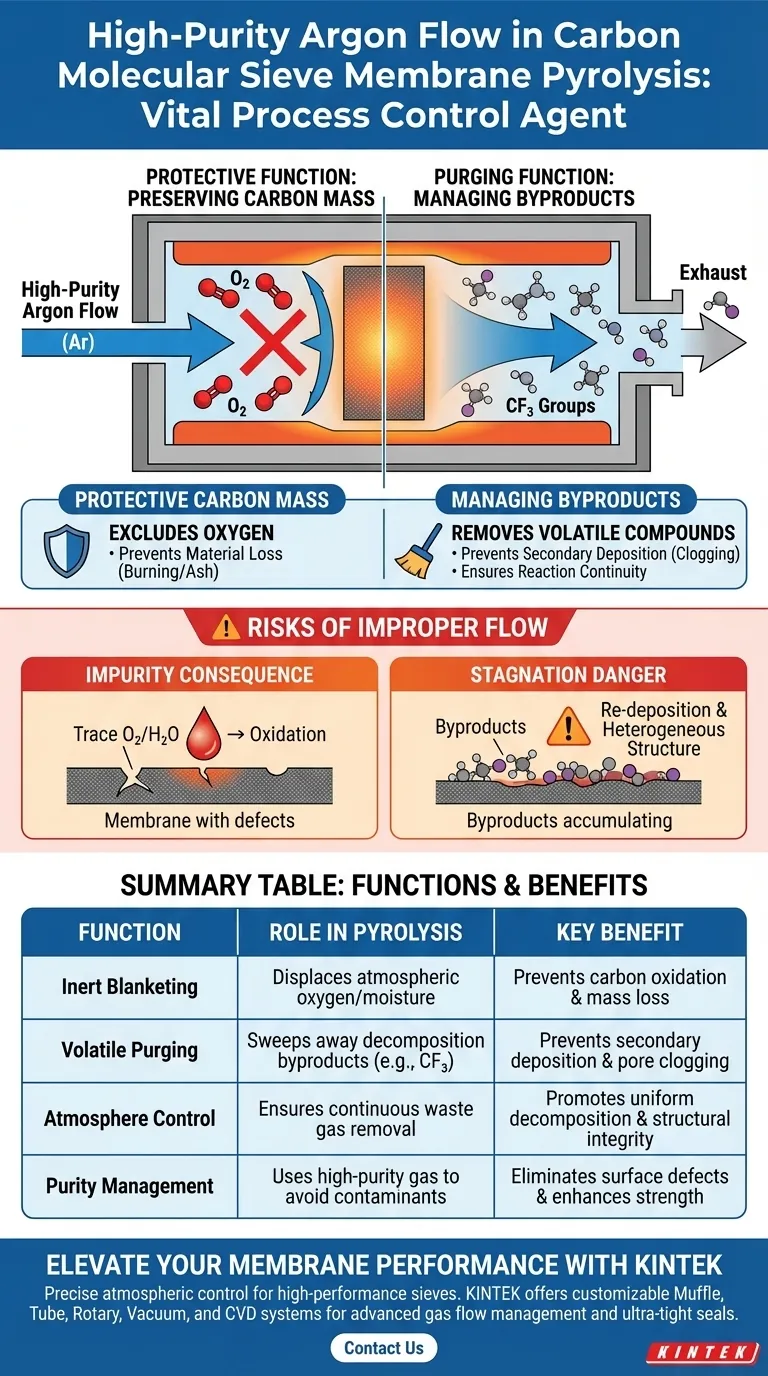
High-purity argon acts as a vital process control agent during the pyrolysis of carbon molecular sieve membranes. It serves two distinct but fundamental purposes: protecting the material from oxidative destruction and actively purging volatile decomposition byproducts to ensure structural integrity.
The success of pyrolysis hinges on maintaining an inert atmosphere; argon flow prevents the combustion of carbon material while sweeping away reactive gases that could compromise the membrane's final pore structure.

The Protective Function: Preserving Carbon Mass
Excluding Oxygen from the Chamber
The primary threat to carbon molecular sieve membranes during high-temperature processing is oxidation.
High-purity argon creates an inert blanket within the furnace chamber. This effectively displaces atmospheric oxygen, which is critical because carbon is highly reactive at pyrolysis temperatures.
Preventing Material Loss
Without the argon barrier, the carbon precursor would essentially burn rather than carbonize.
Oxygen exposure leads to the formation of carbon dioxide or carbon monoxide, resulting in significant loss of carbon mass. Argon ensures that the polymer precursor converts into the desired carbon structure rather than turning into ash.
The Purging Function: Managing Reaction Byproducts
Removal of Volatile Compounds
As the polymer precursor decomposes, it releases various volatile gases.
The continuous flow of argon actively sweeps these byproducts out of the reaction zone. This is particularly important for removing specific decomposition products, such as those derived from CF3 groups, which are released as the polymer breaks down.
Preventing Secondary Deposition
If volatile byproducts are allowed to linger in the furnace, they can interfere with the membrane's quality.
Stagnant gases can lead to secondary deposition, where decomposition products settle back onto the membrane surface. This unwanted deposition can clog pores or alter the surface chemistry, degrading the separation performance of the final sieve.
Ensuring Reaction Continuity
The flow of argon facilitates the intended chemical pathway of pyrolysis.
By constantly removing the "waste" gases generated by the reaction, argon ensures the environment remains conducive to the continued, uniform decomposition of the remaining polymer material.
Understanding the Risks of Improper Flow
The Consequence of Impurity
The specification of "high-purity" argon is not a suggestion; it is a requirement.
Even trace amounts of oxygen or moisture in the gas supply can initiate oxidation at peak temperatures. Using industrial-grade argon with lower purity levels often results in surface defects or reduced mechanical strength in the final membrane.
The Danger of Stagnation
A static inert atmosphere is insufficient; the gas must be flowing.
If the flow rate is too low, volatile byproducts will accumulate near the membrane surface. This increases the likelihood of re-deposition, resulting in a heterogeneous structure that behaves unpredictably during gas separation applications.
Making the Right Choice for Your Goal
To optimize your pyrolysis process, you must view argon flow as a critical variable rather than a passive utility.
- If your primary focus is preventing mass loss: Ensure the argon supply is certified high-purity to eliminate all traces of oxygen within the furnace chamber.
- If your primary focus is pore structure and consistency: Maintain a sufficient and constant flow rate to aggressively sweep away volatile decomposition products like CF3 groups preventing secondary deposition.
Control the atmosphere, and you control the quality of the carbon molecular sieve.
Summary Table:
| Function | Role in Pyrolysis | Key Benefit |
|---|---|---|
| Inert Blanketing | Displaces atmospheric oxygen and moisture | Prevents carbon oxidation and material mass loss |
| Volatile Purging | Sweeps away decomposition byproducts (e.g., CF3 groups) | Prevents secondary deposition and pore clogging |
| Atmosphere Control | Ensures continuous removal of waste gases | Promotes uniform decomposition and structural integrity |
| Purity Management | Uses high-purity gas to avoid trace contaminants | Eliminates surface defects and enhances mechanical strength |
Elevate Your Membrane Performance with KINTEK
Precise atmospheric control is the difference between a high-performance sieve and a failed batch. At KINTEK, we specialize in the high-temperature furnace technology required for delicate carbonization processes.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable for your unique pyrolysis needs. Whether you require advanced gas flow management to prevent secondary deposition or ultra-tight seals to maintain high-purity argon environments, our lab high-temp furnaces provide the stability your research demands.
Ready to optimize your carbon molecular sieve production? Contact us today to discuss your custom furnace solution.
Visual Guide
References
- Shan Xu, Yunlong Ji. High‐Performance Carbon Capture with Fluorine‐Tailored Carbon Molecular Sieve Membranes. DOI: 10.1002/adma.202420477
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What is the significance of preheating UHPC molds? Ensure Safety & Longevity with High-Temp Furnaces
- Why are a blast drying oven and a freeze dryer both necessary for GO nanofibers? Essential Drying Synergy
- Why is a vibratory mill used for ultra-fine grinding when preparing magnesite samples for zeta potential tests?
- What is the primary function of a high-precision drop furnace? Master Flash Smelting Simulation Kinetics
- What are the advantages of the re-coating process? Boost Adsorbent Capacity Beyond Original Performance
- What is the primary value of using a thermal simulator for weather-resistant steel? Precision Hot Working Modeling
- Why is a vacuum drying apparatus necessary for iridium salt precursor impregnation? Unlock Superior Template Loading
- What role does the impregnation method play when using cordierite as a carrier? Enhance Catalyst Loading & Activity



















