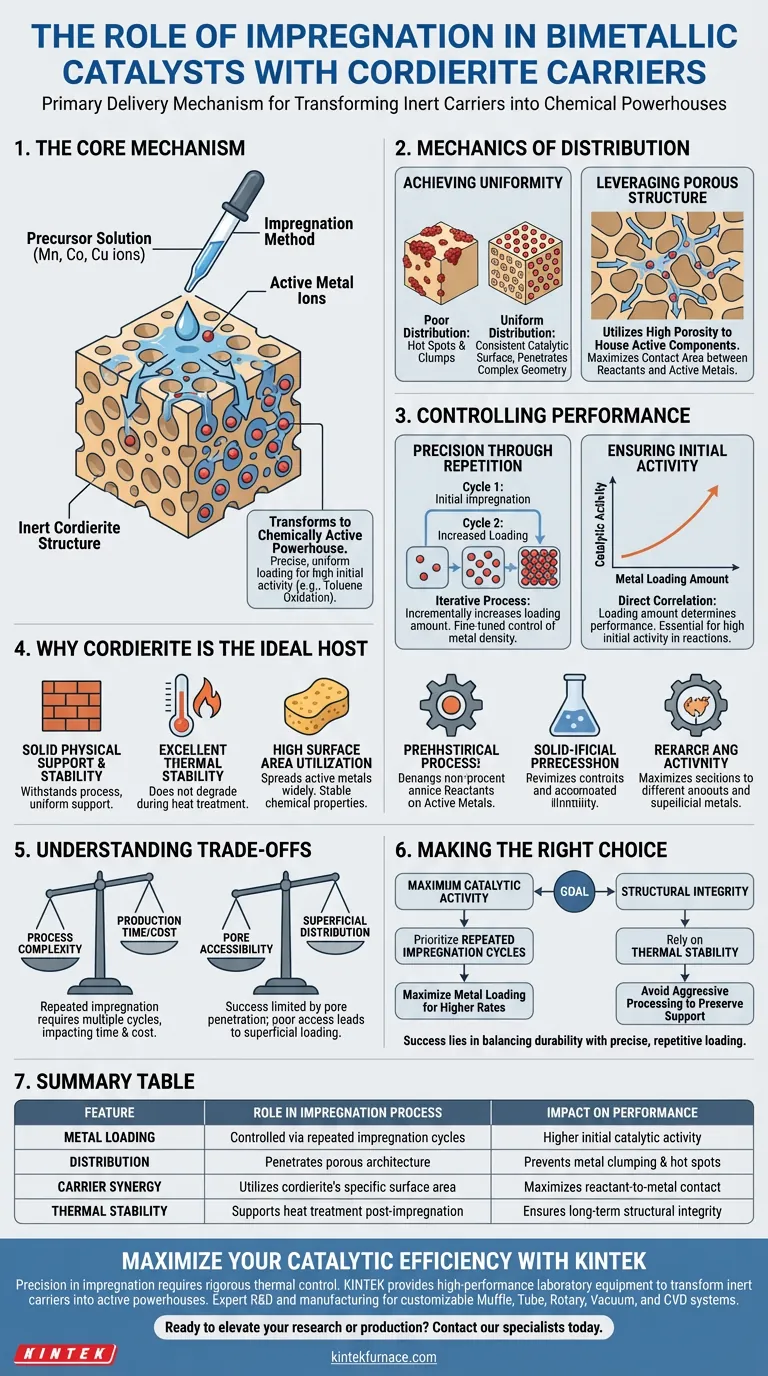
The impregnation method functions as the primary delivery mechanism for introducing active metal components into a cordierite carrier. It is the specific process used to absorb precursor solutions containing ions—such as manganese, cobalt, or copper—and anchor them deep within the carrier's porous architecture. By managing this process, you determine the ultimate distribution and density of the bimetallic catalyst.
The core purpose of the impregnation method is to transform the inert cordierite structure into a chemically active powerhouse. It allows for the precise, uniform loading of metal ions, which is critical for ensuring the high initial activity required for reactions like toluene oxidation.
The Mechanics of Distribution
Achieving Uniformity
The primary goal of impregnation is uniform distribution. Simply applying metal to the surface is insufficient for high-performance catalysis.
The method ensures that precursor solutions penetrate the complex geometry of the carrier. This prevents "hot spots" or clumps of metal, creating a consistent catalytic surface.
Leveraging the Porous Structure
Cordierite is valuable because of its porous structure. The impregnation method utilizes this porosity to house the active components.
By soaking the solution into these pores, the method maximizes the contact area between the reactants and the active metals.
Controlling Catalyst Performance
Precision Through Repetition
A single pass is often not enough to achieve the desired catalytic potency. The reference highlights the importance of repeated impregnation.
This iterative process allows you to incrementally increase the loading amount of active metal components. It gives you fine-tuned control over exactly how much metal is deposited on the surface.
Ensuring Initial Activity
The loading amount directly correlates to performance. By controlling the loading via impregnation, you ensure the initial activity of the catalyst.
This is specifically noted to be effective for applications such as toluene oxidation, where sustained chemical activity is required.
Why Cordierite is the Ideal Host
Physical Support and Stability
The impregnation method relies on the carrier's ability to withstand the process. Cordierite provides a solid and uniform physical support.
Its excellent thermal stability ensures it does not degrade during the heat treatment often required after impregnation.
Surface Area Utilization
Cordierite is chosen for its high specific surface area utilization. The impregnation method exploits this characteristic to spread the active metals as widely as possible.
Furthermore, its stable chemical properties prevent unwanted reactions between the carrier and the precursor solutions during the impregnation phase.
Understanding the Trade-offs
Process Complexity
While effective, the requirement for repeated impregnation introduces complexity. Achieving high loading amounts is not instantaneous.
It requires multiple cycles to build up the necessary density of active components, which can impact production time and cost.
Dependency on Pore Accessibility
The success of the method is strictly limited by the porous structure of the cordierite.
If the solution cannot penetrate the pores effectively, the distribution will remain superficial, negating the benefits of the carrier's internal surface area.
Making the Right Choice for Your Goal
To optimize your bimetallic catalyst, consider your specific performance targets:
- If your primary focus is Maximum Catalytic Activity: Prioritize repeated impregnation cycles to maximize the loading amount of manganese, cobalt, or copper ions for higher oxidation rates.
- If your primary focus is Structural Integrity: Rely on Cordierite’s thermal stability and avoid aggressive processing that might compromise the physical support of the carrier.
Success lies in balancing the durability of the cordierite support with the precise, repetitive loading of active metals.
Summary Table:
| Feature | Role in Impregnation Process | Impact on Performance |
|---|---|---|
| Metal Loading | Controlled via repeated impregnation cycles | Higher initial catalytic activity |
| Distribution | Penetrates porous architecture | Prevents metal clumping & hot spots |
| Carrier Synergy | Utilizes cordierite's specific surface area | Maximizes reactant-to-metal contact |
| Thermal Stability | Supports heat treatment post-impregnation | Ensures long-term structural integrity |
Maximize Your Catalytic Efficiency with KINTEK
Precision in impregnation requires rigorous thermal control. KINTEK provides the high-performance laboratory equipment necessary to transform inert carriers into active chemical powerhouses. Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique catalyst synthesis needs.
Ready to elevate your research or production? Contact our specialists today to find the perfect high-temp furnace solution for your laboratory.
Visual Guide
References
- Xiaojian Wang, Hao Huang. Synergistic oxidation of toluene through bimetal/cordierite monolithic catalysts with ozone. DOI: 10.1038/s41598-024-58026-6
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
People Also Ask
- How are electric furnaces applied in powder metallurgy and 3D printing? Unlock Precision Sintering and Heat Treatment
- Why is a heating system with closed-loop feedback essential for TL analysis? Precision Tips for High-Accuracy Kinetics
- What factors should be considered when selecting a furnace based on material properties? Ensure Optimal Heat Treatment
- How does calcination temperature affect CuO grain growth? Optimize Nanoporous Film Morphology and Crystallinity
- What are the benefits of thermal cycling furnaces? Boost Speed, Efficiency, and Durability
- How does a constant temperature drying oven contribute to MgTiO3-CaTiO3 ceramic slurry? Optimize Your Precursor Quality
- What is the role of a forced convection oven in DPKB-S preparation? Optimize Biochar Synthesis and Material Purity
- What specific information does SEM provide for LFP synthesis? Master Battery-Grade Quality Control
