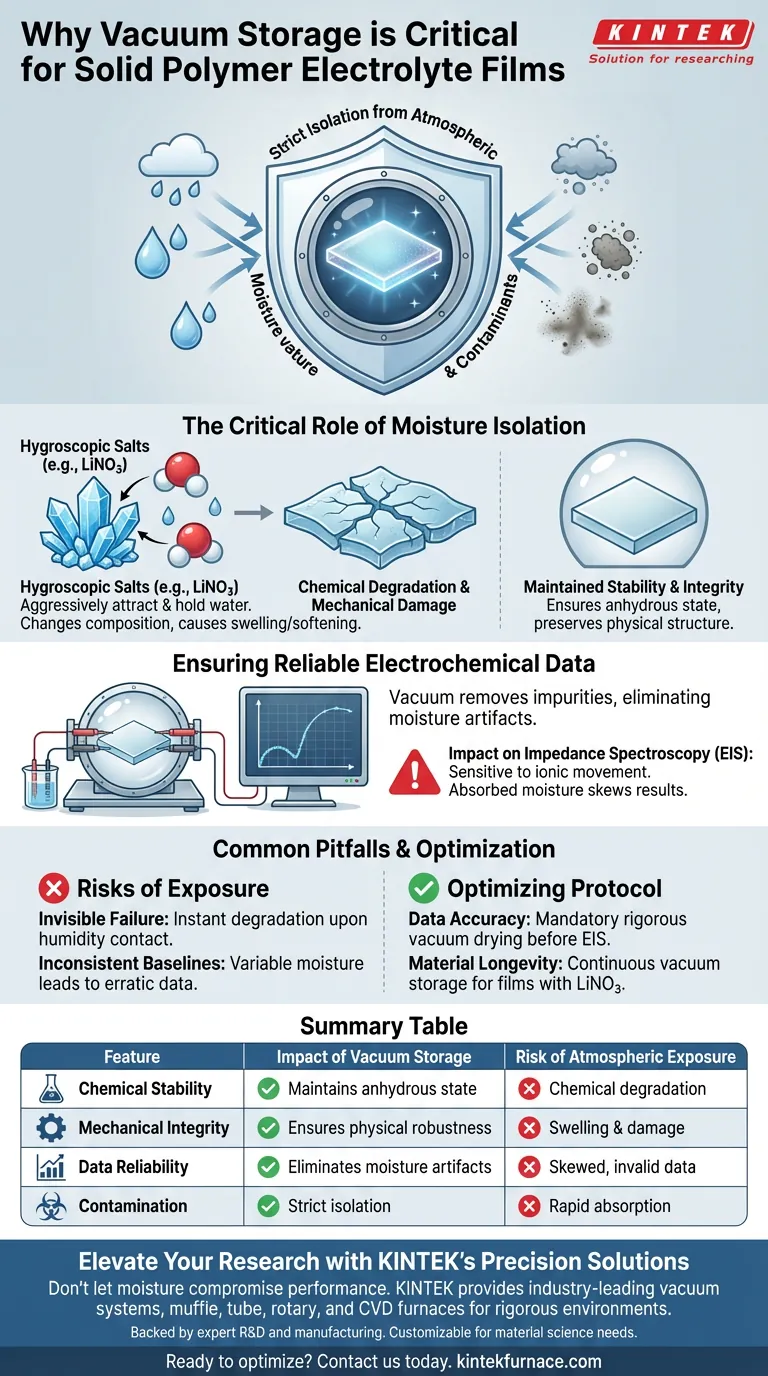
A vacuum storage environment is critical for the successful formation and maintenance of solid polymer electrolyte films because it strictly isolates the material from atmospheric moisture and contaminants. This isolation is strictly necessary to prevent water absorption—specifically in films containing hygroscopic salts like Lithium Nitrate (LiNO3)—which ensures the film retains the mechanical integrity and chemical stability required for accurate performance testing.
Vacuum environments act as a mandatory shield against environmental interference, preventing moisture-induced degradation that would otherwise render electrochemical data invalid and compromise the physical structure of the polymer.

The Critical Role of Moisture Isolation
Managing Hygroscopic Components
Many solid polymer electrolytes incorporate salts, such as Lithium Nitrate (LiNO3), to enhance conductivity or stability. These salts are often hygroscopic, meaning they aggressively attract and hold water molecules from the surrounding air.
preventing Chemical Degradation
When these salts absorb atmospheric moisture, the chemical composition of the electrolyte changes immediately. A vacuum environment effectively eliminates this risk, maintaining the chemical stability of the film by ensuring the components remain in their intended anhydrous state.
Preserving Mechanical Integrity
Moisture absorption does not just alter chemistry; it changes the physical structure. Water uptake can cause swelling or softening, damaging the mechanical integrity of the film. Vacuum storage ensures the film remains physically robust and dimensionally stable.
Ensuring Reliable Data
The Impact on Impedance Spectroscopy
Researchers rely on Electrochemical Impedance Spectroscopy (EIS) to characterize the performance of these films. This testing method is extremely sensitive to ionic movement and resistance.
Removing Experimental Variables
If a film contains absorbed moisture, the water molecules participate in the electrochemical reactions, skewing the results. Vacuum drying and storage remove these impurities, ensuring that the EIS data reflects the true properties of the polymer, not the contaminants.
Common Pitfalls of Environmental Exposure
The Risk of "Invisible" Failure
A major oversight is assuming that brief exposure to air is harmless. For hygroscopic materials, performance degradation begins almost instantly upon contact with humidity.
Inconsistency in Research Baselines
Without strict vacuum protocols, it is impossible to establish a reliable baseline for experimentation. Variable moisture levels lead to erratic data, making it difficult to distinguish between a failed film formulation and a film ruined by atmospheric impurities.
Optimizing Your Storage Protocol
To ensure the validity of your electrochemical research, you must treat the storage environment as a variable as critical as the chemical formulation itself.
- If your primary focus is data accuracy: rigorous vacuum drying is mandatory prior to any EIS testing to eliminate moisture artifacts.
- If your primary focus is material longevity: store all films containing LiNO3 in a continuous vacuum environment to prevent cumulative degradation over time.
By strictly controlling the atmosphere around your polymer electrolytes, you transform a variable process into a reliable, reproducible science.
Summary Table:
| Feature | Impact of Vacuum Storage | Risk of Atmospheric Exposure |
|---|---|---|
| Chemical Stability | Maintains anhydrous state for hygroscopic salts like LiNO3 | Chemical degradation and altered composition |
| Mechanical Integrity | Ensures physical robustness and dimensional stability | Swelling, softening, and physical structure damage |
| Data Reliability | Eliminates moisture artifacts for accurate EIS testing | Skewed electrochemical results and invalid data |
| Contamination | Strict isolation from atmospheric impurities | Rapid absorption of moisture and environmental contaminants |
Elevate Your Research with KINTEK’s Precision Solutions
Don't let atmospheric moisture compromise your electrolyte performance or research validity. KINTEK provides industry-leading laboratory equipment designed to maintain the rigorous environments your materials demand.
Backed by expert R&D and manufacturing, KINTEK offers vacuum systems, muffle, tube, rotary, and CVD furnaces, all customizable for your unique material science needs. Ensure your solid polymer films retain their mechanical integrity and chemical stability with our high-performance thermal and vacuum solutions.
Ready to optimize your lab's workflow? Contact us today to discuss how our customizable systems can support your specific research goals.
Visual Guide
References
- Mohan Srinivas, R. F. Bhajantri. Strategy on enhancing ionic conductivity of biocompatible hydroxypropylmethylcellulose/polyethylene glycol polymer blend electrolyte with TiO2 nanofillers and LiNO3 ionic salt. DOI: 10.5599/jese.2351
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
People Also Ask
- Why is immediate water quenching required for CuAlMn alloys? Master Phase Retention in Shape Memory Alloys
- What is the role of high-purity helium in electromagnetic levitation? Key for Rapid Thermal Regulation
- How does diamond benefit 5G technology? Unlock Peak Performance with Superior Thermal Management
- How does ALD of Li2CO3 contribute to NMC thin film performance? Restore Battery Capacity with Precision
- What are the functions of hydrogen gas for graphene on silver? Enhance Crystallinity & Stability
- What are the advantages of using a precision vacuum drying oven? Master Ceramic Powder Treatment with KINTEK
- Why is high-temperature hydrogen reduction used for HI decomposition catalysts? Boost Efficiency and Surface Purity
- What critical environmental conditions does a high-temperature recrystallization annealing furnace provide? Maximize Steel Strength












