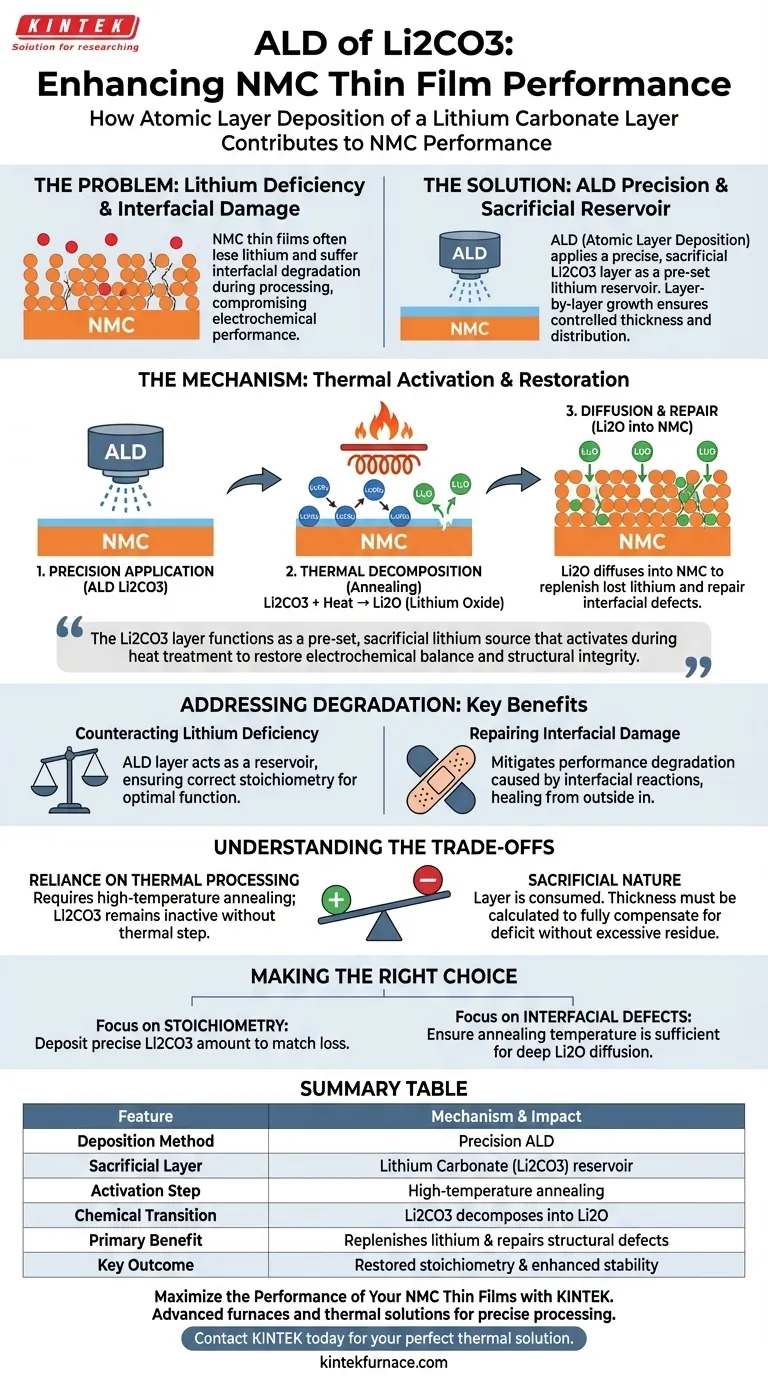
Atomic Layer Deposition (ALD) serves as a precise remediation tool for NMC thin films by creating a sacrificial lithium carbonate (Li2CO3) reservoir. When the film undergoes high-temperature annealing, this layer decomposes into lithium oxide (Li2O), which diffuses back into the NMC material to replenish lost lithium and repair structural degradation caused by interfacial reactions.
The Li2CO3 layer functions as a pre-set, sacrificial lithium source that activates during heat treatment. By compensating for lithium loss and repairing interfacial damage, it restores the electrochemical balance and structural integrity of the NMC thin film.

The Mechanism of Lithium Restoration
Precision Application
ALD allows for the creation of a sacrificial Li2CO3 layer on the surface of the NMC thin film.
Because ALD utilizes highly controlled, layer-by-layer growth, the thickness and distribution of this lithium source can be tuned with extreme accuracy.
Thermal Decomposition
The restoration process is triggered during subsequent high-temperature annealing.
Under this heat, the pre-set Li2CO3 layer decomposes. This chemical reaction transforms the carbonate into lithium oxide (Li2O).
Diffusion and Repair
The newly formed Li2O does not remain on the surface; it diffuses back into the NMC film.
This diffusion targets areas within the film that are lithium-deficient. It effectively compensates for lithium loss that occurred during previous processing steps.
Addressing Material Degradation
Counteracting Lithium Deficiency
NMC thin films are prone to losing lithium, which compromises their electrochemical performance.
The ALD-deposited layer acts as a reservoir, ensuring that the final material maintains the correct stoichiometry required for optimal function.
Repairing Interfacial Damage
Beyond simple replenishment, this process actively repairs material defects.
The diffusion of Li2O helps mitigate performance degradation specifically caused by interfacial reactions, healing the film's structure from the outside in.
Understanding the Trade-offs
Reliance on Thermal Processing
This is not a passive coating; it is a chemically active process that requires heat to function.
The benefits of the Li2CO3 layer are only realized during the high-temperature annealing phase. Without this thermal step, the layer would remain as carbonate and fail to release the necessary Li2O for diffusion.
The Sacrificial Nature
The Li2CO3 layer is designed to be consumed, not to remain as a permanent barrier.
Engineers must calculate the deposition thickness carefully. The goal is to provide enough material to compensate for the specific deficit within the NMC film without leaving excessive residue or failing to fully repair the deficiency.
Making the Right Choice for Your Goal
To maximize the performance of your NMC thin films, consider how this technique aligns with your processing requirements:
- If your primary focus is correcting stoichiometry: Utilize ALD to deposit a precise amount of Li2CO3 calculated to match the expected lithium loss during fabrication.
- If your primary focus is repairing interfacial defects: Ensure your post-deposition annealing temperatures are sufficient to fully decompose the Li2CO3 and drive the diffusion of Li2O deep into the film.
By treating the Li2CO3 layer as an active reactant rather than a passive coating, you ensure the long-term stability and efficiency of the final cathode material.
Summary Table:
| Feature | Mechanism & Impact |
|---|---|
| Deposition Method | Precision ALD (Atomic Layer Deposition) |
| Sacrificial Layer | Lithium Carbonate (Li2CO3) reservoir |
| Activation Step | High-temperature annealing phase |
| Chemical Transition | Li2CO3 decomposes into Lithium Oxide (Li2O) |
| Primary Benefit | Replenishes lithium loss and repairs structural defects |
| Key Outcome | Restored stoichiometry and enhanced electrochemical stability |
Maximize the Performance of Your NMC Thin Films
Precise control over lithium stoichiometry is vital for high-performance battery research. KINTEK provides the advanced technology needed to achieve these results. Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, as well as other lab high-temperature furnaces—all fully customizable to meet your unique thermal processing and annealing needs.
Ready to enhance your material stability and battery efficiency? Contact KINTEK today to find the perfect thermal solution for your laboratory.
Visual Guide
References
- Sameer R.J. Rodrigues, Philippe M. Vereecken. Coupled Solid‐State Diffusion of Li<sup>+</sup> and O<sup>2 −</sup> During Fabrication of Ni‐Rich NMC Thin‐Film Cathodes Resulting in the Formation of Inactive Ni<sub>2</sub>O<sub>3</sub> and NiO Phases. DOI: 10.1002/admi.202400911
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Inclined Rotary Plasma Enhanced Chemical Deposition PECVD Tube Furnace Machine
- Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Inclined Rotary Plasma Enhanced Chemical Deposition PECVD Tube Furnace Machine
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
People Also Ask
- What role does a reactive atmosphere like nitrogen play in PFS? Enhance Titanium Dioxide Surface Treatment
- What are the advantages of a benchtop industrial oven in terms of usability? Enhance Lab Efficiency with Compact Design
- What is the design focus of a thermal reactor in flash pyrolysis? Optimize Bio-oil Yield with Precision Engineering
- What is the role of carbonaceous reducing agents in copper slag treatment? Maximize Metal Recovery with Expert Insights
- What is the disadvantage of dental ceramic? Weighing Cost, Strength, and Aesthetics
- How do Digital Twin and machine learning improve maintenance? Master High-Temp Equipment Reliability & Efficiency
- How does a carbonization furnace control the microstructural properties of a macroporous carbon framework (MPCF)?
- What is the mechanism of high-power microwave systems in uranium roasting? Unlock Efficiency with Volumetric Heating



















