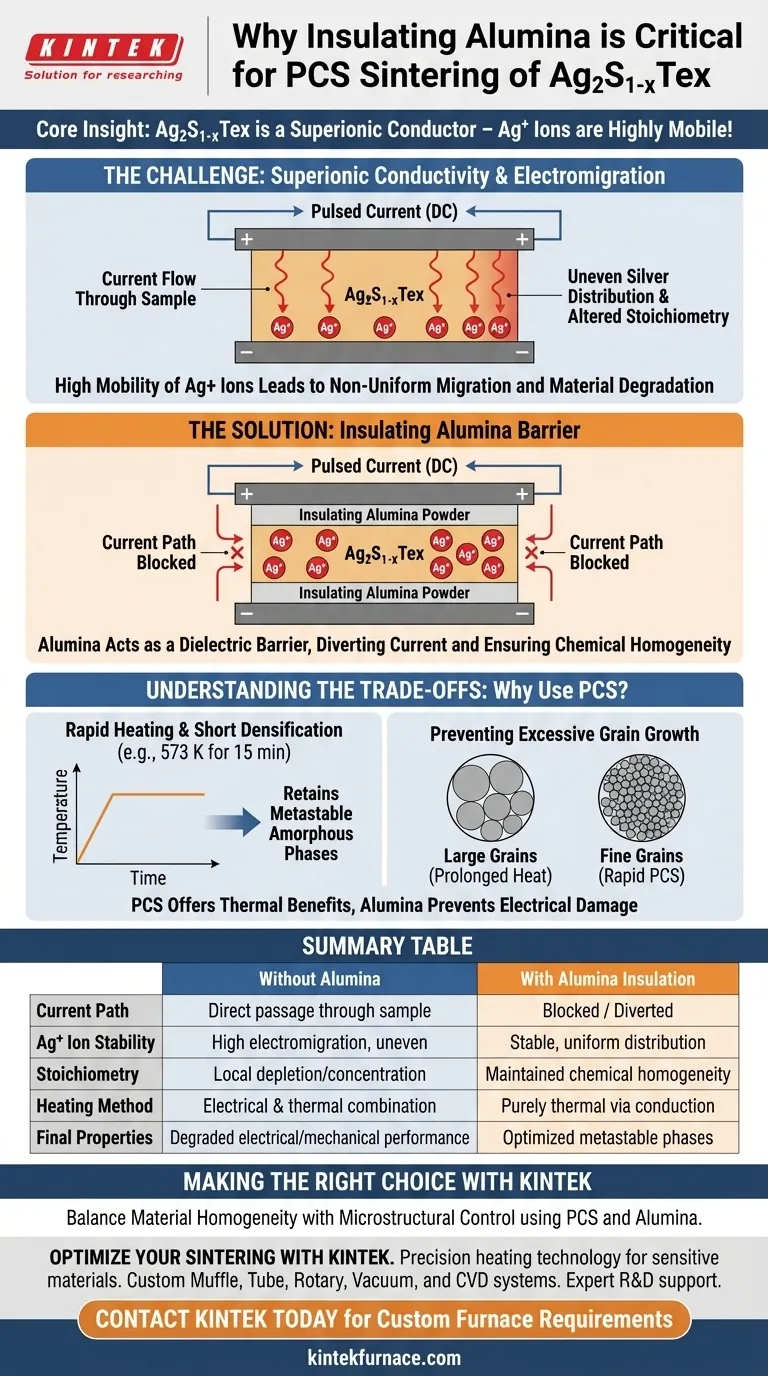
The use of insulating alumina powder is a critical requirement to block the direct passage of electric current through Ag2S1-xTex samples during the sintering process. Without this insulating barrier, the strong direct current used in Pulsed Current Sintering (PCS) would drive the silver ions to migrate unevenly, destroying the material's structural and chemical homogeneity.
Core Insight: Ag2S1-xTex acts as a superionic conductor, meaning its silver ions are highly mobile and susceptible to electric fields. Alumina insulation effectively isolates the material from the current, ensuring that the densification process occurs without triggering destructive ion migration.

The Challenge of Superionic Conductivity
High Mobility of Silver Ions
The material Ag2S1-xTex is not a standard ceramic; it possesses superionic conductivity.
In this state, silver ions (Ag+) are loosely bound and can move with exceptional freedom within the lattice structure.
The Risk of Electromigration
When a material with superionic properties is exposed to a strong direct current (DC), the ions do not stay static.
The electric field applies a force to the charged Ag+ ions, causing them to migrate physically toward the negative electrode.
This non-uniform migration depletes silver in some areas and concentrates it in others, altering the local stoichiometry of the sample.
The Role of Insulating Alumina
Blocking the Current Path
To prevent this migration, the sample is covered on the top and bottom with insulating alumina powder.
This powder acts as a dielectric barrier, effectively blocking the direct passage of current through the Ag2S1-xTex material.
Ensuring Homogeneity
By diverting the current away from the sample, the alumina ensures the silver ions remain evenly distributed.
This preservation of internal structure is vital for ensuring the final sintered part maintains consistent electrical and mechanical properties.
Understanding the Trade-offs: Why Use PCS?
The Necessity of Rapid Heating
You might wonder why PCS is used at all if the current poses such a risk to the silver ions.
The answer lies in the supplementary benefit of PCS: extremely fast heating rates and short densification times.
Retaining Metastable Phases
Prolonged exposure to high temperatures typically results in excessive grain growth, which degrades material performance.
The rapid sintering of PCS (e.g., 573 K for just 15 minutes) maximizes the retention of metastable amorphous phases.
Balancing Process and Chemistry
The alumina powder represents a necessary compromise.
It allows engineers to utilize the rapid thermal benefits of PCS equipment without subjecting the sensitive superionic material to the destructive effects of the DC current that drives the machine.
Making the Right Choice for Your Goal
When processing silver-based chalcogenides like Ag2S1-xTex, balancing the thermal method with electrical isolation is key.
- If your primary focus is Material Homogeneity: You must use insulating alumina powder to prevent the electric field from driving non-uniform silver ion migration.
- If your primary focus is Microstructural Control: You should utilize the PCS method to achieve rapid densification and prevent excessive grain growth, provided the sample is electrically isolated.
By isolating the sample electrically while utilizing the rapid thermal application of PCS, you secure both the chemical integrity and the microstructural advantages necessary for high-performance materials.
Summary Table:
| Feature | Impact on Ag2S1-xTex without Alumina | Impact with Alumina Insulation |
|---|---|---|
| Current Path | Direct passage through sample | Blocked / Diverted from sample |
| Ag+ Ion Stability | High electromigration toward electrodes | Stable, uniform distribution |
| Stoichiometry | Local depletion and concentration | Maintained chemical homogeneity |
| Heating Method | Electrical & thermal combination | Purely thermal via conduction |
| Final Properties | Degraded electrical/mechanical performance | Optimized metastable phases |
Optimize Your Advanced Material Sintering with KINTEK
Precision is paramount when handling sensitive superionic conductors like silver-based chalcogenides. KINTEK provides the high-performance heating technology required to balance rapid densification with chemical integrity. Backed by expert R&D and world-class manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique laboratory needs.
Whether you are refining PCS protocols or require specialized high-temperature furnaces, our experts are ready to assist. Contact KINTEK today to discuss your custom furnace requirements and ensure your materials achieve peak performance.
Visual Guide
References
- Kosuke Sato, Tsunehiro Takeuchi. Composition, time, temperature, and annealing-process dependences of crystalline and amorphous phases in ductile semiconductors Ag2S1−<i>x</i>Te<i>x</i> with <i>x</i> = 0.3–0.6. DOI: 10.1063/5.0180950
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Molybdenum Vacuum Heat Treat Furnace
People Also Ask
- What is the design basis of SCR Type Silicon Carbide Heating Elements? Optimize for Precision Control
- How does a graphite heater work? Achieving Extreme Temperatures Beyond 2000°C
- What are the limitations of Copper Nickel alloys for heating applications? Key Temperature and Performance Insights
- How is molybdenum disilicide used in microelectronics? Boost Chip Speed with MoSi₂ Shunts
- What are the key properties and applications of MoSi2 heating elements? Unlock High-Temperature Performance
- What materials are typically used in the construction of high temperature heating elements? Discover the Best Options for Your Needs
- What are the main characteristics of silicon carbide heating elements compared to metal heating elements? Discover Key Differences for Your High-Temp Needs
- What is a band heater and how is it used? Boost Efficiency in Industrial Heating



















