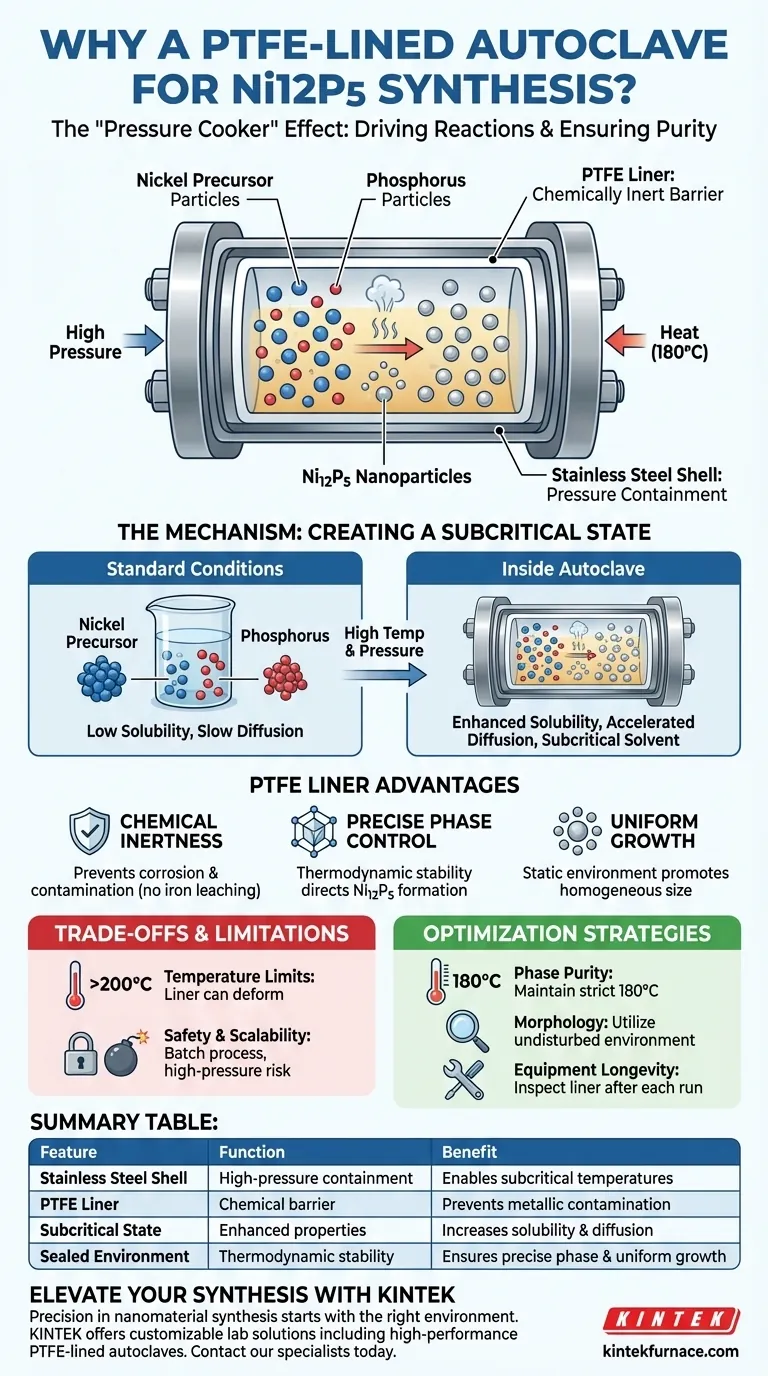
The PTFE-lined stainless steel autoclave is the standard vessel for creating the specific subcritical conditions necessary to synthesize nickel phosphide (Ni12P5). This device seals the reaction mixture, allowing it to reach temperatures (typically 180°C) and pressures well above the solvent's boiling point. This environment dramatically enhances the solubility and diffusion of the nickel and phosphorus sources, driving a reaction that would be kinetically impossible at atmospheric pressure.
Core Takeaway: The autoclave creates a "pressure cooker" effect that forces otherwise insoluble precursors to dissolve and react, while the PTFE liner ensures the delicate chemical environment remains chemically inert and free from metallic contamination.
The Mechanism of Solvothermal Synthesis
Creating a Subcritical State
The primary function of the stainless steel shell is to withstand high pressure. By sealing the reactants and heating them to 180°C, the solvent enters a subcritical state.
In this state, the physical properties of the solvent change drastically. It allows the liquid to remain fluid at temperatures where it would normally boil away, facilitating high-energy interactions.
Enhancing Solubility and Diffusion
Under standard ambient conditions, nickel and red phosphorus sources often struggle to mix effectively. The high pressure within the autoclave significantly increases the solubility of these reactants.
Simultaneously, the elevated temperature accelerates diffusion rates. This ensures the reactants meet and interact thoroughly in the liquid phase, leading to a complete chemical reaction.
The Importance of the PTFE Liner
Ensuring Chemical Inertness
While the steel provides structural integrity, it is chemically reactive. The polytetrafluoroethylene (PTFE) liner acts as a crucial barrier between the reaction solution and the steel body.
This prevents the reaction from corroding the steel, which is vital when using corrosive solvents or precursors. It also prevents iron from the steel leaching into your solution and contaminating the purity of the Ni12P5.
Facilitating Precise Phase Control
The "black box" environment of the autoclave allows for precise thermodynamic control. This stability is required to direct the reaction toward the specific Ni12P5 phase, rather than other potential nickel phosphide stoichiometries.
Promoting Uniform Growth
The static, sealed environment suppresses the turbulence found in stirred reactors. This facilitates the uniform growth of nanoparticles.
By maintaining consistent pressure and temperature gradients, the autoclave ensures the resulting nanoparticles possess a homogeneous size and morphology.
Understanding the Trade-offs
Temperature Limitations
While excellent for chemical resistance, PTFE has thermal limits. It can soften or deform if the temperature significantly exceeds 200°C–220°C, potentially compromising the seal.
Safety and Scalability
These autoclaves act as high-pressure bombs if mishandled. They generally rely on batch processing, making them difficult to scale up for mass production compared to continuous flow reactors.
Optimizing Your Synthesis Strategy
- If your primary focus is Phase Purity: Ensure your temperature is strictly maintained at 180°C to utilize the subcritical state for the correct Ni12P5 stoichiometry.
- If your primary focus is Morphology: Rely on the undisturbed nature of the sealed autoclave to promote uniform crystal growth without mechanical agitation.
- If your primary focus is Equipment Longevity: Inspect the PTFE liner after every run for deformation or contamination to protect the expensive stainless steel shell.
The autoclave effectively separates the mechanical requirement of pressure containment from the chemical requirement of reaction purity, creating the ideal environment for advanced nanomaterial synthesis.
Summary Table:
| Feature | Function in Ni12P5 Synthesis | Benefit |
|---|---|---|
| Stainless Steel Shell | High-pressure containment | Enables temperatures above solvent boiling point |
| PTFE Liner | Chemical barrier | Prevents metallic contamination and corrosion |
| Subcritical State | Enhanced solvent properties | Increases solubility and diffusion of Ni and P |
| Sealed Batch Environment | Thermodynamic stability | Ensures precise phase control and uniform growth |
Elevate Your Material Synthesis with KINTEK
Precision in nanomaterial synthesis starts with the right environment. Backed by expert R&D and manufacturing, KINTEK offers a wide range of lab solutions, including high-performance PTFE-lined autoclaves, Muffle, Tube, Rotary, Vacuum, and CVD systems. Our equipment is fully customizable to meet your unique research needs, ensuring the chemical purity and structural integrity of your samples.
Ready to optimize your lab's high-temperature and high-pressure workflows? Contact our specialists today to find your custom solution.
Visual Guide
References
- Ewa Mijowska, Klaudia Maślana. Highly Porous Carbon Flakes Derived from Cellulose and Nickel Phosphide Heterostructure towards Efficient Electrocatalysis of Oxygen Evolution Reaction. DOI: 10.3390/molecules29020352
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 304 316 Stainless Steel High Vacuum Ball Stop Valve for Vacuum Systems
- Molybdenum Vacuum Heat Treat Furnace
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- Why are high-purity graphite molds essential for the sintering of Tin Selenide (SnSe) alloys? Key to Precise SPS Results
- What is the function of vacuum-sealed quartz jackets in sample encapsulation? Ensure Purity in Material Synthesis
- What is the primary function of an industrial vacuum drying oven in Si-RuO2 catalyst preparation? Achieve Uniformity.
- What is the function of a rotary evaporator in the recovery of formic acid lignin? Preserve Quality & Boost Efficiency
- How does the geometric design of a sample basket affect measurement accuracy in thermogravimetric analysis?
- What substances are prohibited from being introduced into the furnace chamber? Prevent Catastrophic Failure
- Why use high-purity graphite for β-Ga2O3 annealing? Key to Thermal Precision & Safety
- What is the function of a laboratory pellet press in PCM preparation? Optimize Building Energy Storage Materials



















