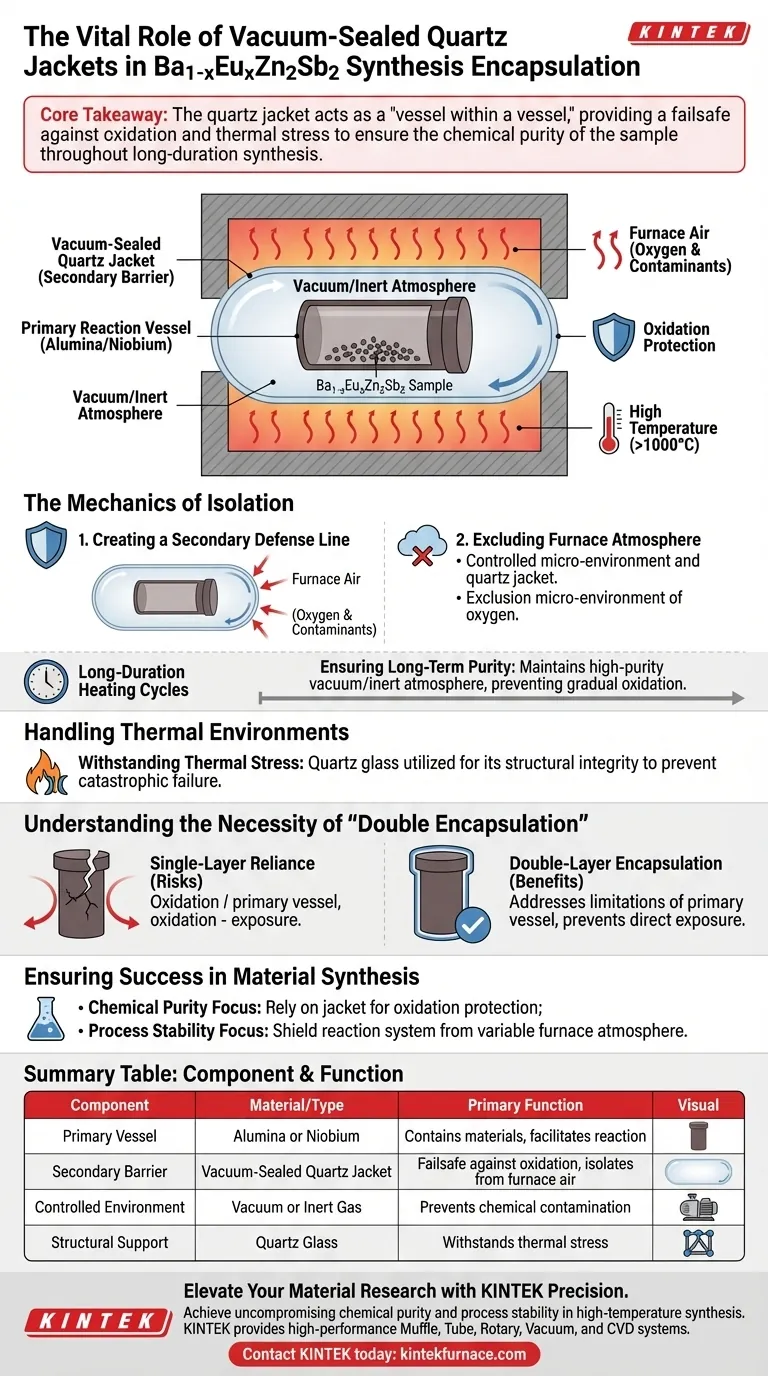
Vacuum-sealed quartz jackets serve as a critical secondary barrier designed to prevent oxidation during the synthesis of $Ba_{1-x}Eu_xZn_2Sb_2$ samples. These jackets encapsulate the primary reaction vessels—typically made of alumina or Niobium—to physically isolate the materials from the ambient air within the furnace. This isolation ensures the reaction proceeds in a high-purity vacuum or inert atmosphere, even during extended high-temperature heating cycles.
Core Takeaway The quartz jacket acts as a "vessel within a vessel," providing a failsafe against oxidation and thermal stress to ensure the chemical purity of the sample throughout long-duration synthesis.

The Mechanics of Isolation
Creating a Secondary Defense Line
In the synthesis of complex materials like $Ba_{1-x}Eu_xZn_2Sb_2$, a single layer of protection is often insufficient. The quartz jacket encloses the primary reaction vessels, such as alumina crucibles or Niobium tubes.
This double-layer approach ensures that even if the primary vessel is permeable or reactive, the sample remains protected.
Excluding Furnace Atmosphere
High-temperature furnaces contain air that can degrade sensitive samples. The vacuum-sealed jacket effectively isolates the reaction system from this external environment.
By creating a controlled micro-environment, the jacket prevents oxygen and other contaminants found in the furnace air from reaching the sample.
Ensuring Long-Term Purity
Synthesis processes often require long-duration heating cycles to achieve the correct crystalline structure.
The quartz jacket maintains a high-purity vacuum or inert atmosphere throughout the entire duration, preventing the gradual oxidation that could occur over time in a less secure setup.
Handling Thermal Environments
Withstanding Thermal Stress
The synthesis of $Ba_{1-x}Eu_xZn_2Sb_2$ involves significant heat generation. The quartz jacket is specifically utilized because it can withstand the thermal stresses associated with these high temperatures.
This structural integrity is vital to prevent catastrophic failure of the containment system during the reaction.
Understanding the Necessity of "Double Encapsulation"
The Risks of Single-Layer Reliance
One might ask why the primary vessel (Alumina or Niobium) is not enough. The quartz jacket addresses the limitations of the primary vessel in isolating the sample from the furnace atmosphere.
Without this secondary vacuum layer, the primary vessel is directly exposed to furnace air, significantly increasing the risk of sample oxidation and contamination.
Ensuring Success in Material Synthesis
To achieve high-quality $Ba_{1-x}Eu_xZn_2Sb_2$ samples, consider the following based on your specific synthesis goals:
- If your primary focus is Chemical Purity: Rely on the vacuum-sealed jacket to provide the necessary oxidation protection that the primary vessel alone cannot guarantee.
- If your primary focus is Process Stability: Utilize the quartz jacket to shield the reaction system from the variable atmosphere of the furnace during long heating cycles.
The vacuum-sealed quartz jacket is not merely a container; it is the guarantor of the inert environment required for successful high-temperature synthesis.
Summary Table:
| Component | Material/Type | Primary Function in Synthesis |
|---|---|---|
| Primary Vessel | Alumina or Niobium | Contains materials and facilitates the initial reaction environment. |
| Secondary Barrier | Vacuum-Sealed Quartz Jacket | Provides a failsafe against oxidation and isolates the reaction from furnace air. |
| Controlled Environment | Vacuum or Inert Gas | Prevents chemical contamination during extended high-temperature heating cycles. |
| Structural Support | Quartz Glass | Withstands thermal stress to prevent containment failure during the process. |
Elevate Your Material Research with KINTEK Precision
Achieve uncompromising chemical purity and process stability in your high-temperature synthesis. Backed by expert R&D and world-class manufacturing, KINTEK provides high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, along with customizable lab furnaces designed to meet your most demanding thermal requirements.
Don't let oxidation or thermal stress compromise your samples. Contact KINTEK today to discover how our advanced heating solutions and vacuum technologies can optimize your laboratory’s efficiency and success.
Visual Guide
References
- Daewon Shim, Tae‐Soo You. Eu-Substituents-Induced Modifications in the Thermoelectric Properties of the Zintl Phase Ba1-xEuxZn2Sb2 System. DOI: 10.3390/molecules30020310
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Vacuum Heat Treat Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- How does a precision programmed cooling system influence the structural integrity of Al2O3-TiC composite materials?
- Why is an alumina crucible necessary for g-C3N4 synthesis? Ensure High Purity & Stability in Polycondensation
- How does excessive gas purging rate affect the alumina furnace tube? Prevent Cracking and Extend Tube Life
- What is the function of a Teflon-lined stainless steel autoclave in the hydrothermal synthesis of Bi2O3 precursors?
- What are the technical advantages of using ZrO2 crucibles? Elevate Smelting Accuracy with Zirconium Dioxide
- Why is a Zirconia (ZrO2) oxygen sensor used for CaO-Al2O3-VOx slag research? Achieve Precise Redox Control
- How does a laboratory vacuum system contribute to high-purity high-entropy alloys? Essential Insights
- Why use non-conductive polymer containers for carbon nanoparticle testing? Ensure Data Integrity and Precision



















