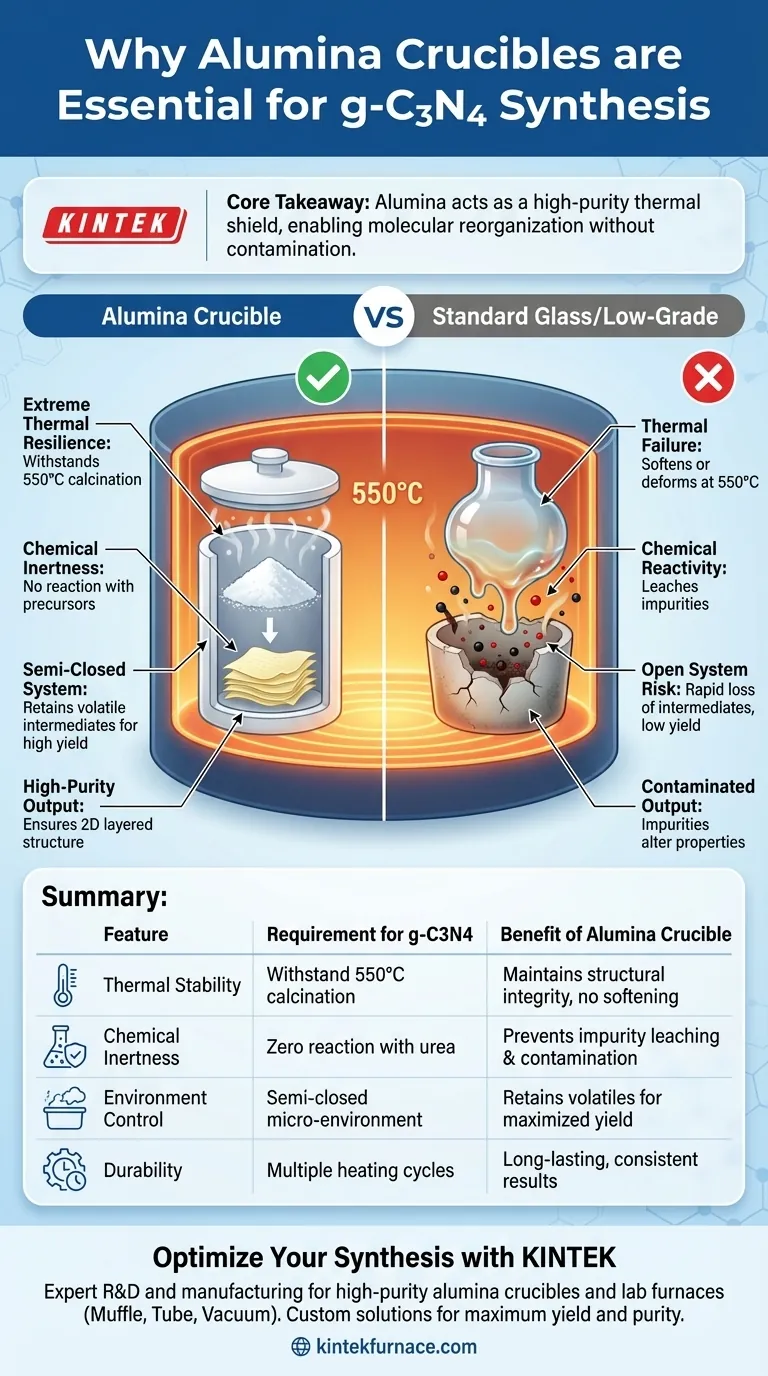
The necessity of an alumina crucible lies in its combination of extreme thermal resilience and chemical inertness. This vessel serves as a stable containment unit capable of withstanding the 550°C calcination temperatures required for the synthesis without physical degradation. More importantly, alumina remains chemically non-reactive during the decomposition of urea, ensuring that no impurities leach from the container into the final graphitic carbon nitride (g-C3N4) structure.
Core Takeaway: Alumina acts as a high-purity thermal shield that withstands the aggressive environment of polymerization. Its primary function is to facilitate the molecular reorganization of precursors into a layered 2D structure while preventing the introduction of external contaminants.
The Critical Role of Thermal Stability
Withstanding Calcination Temperatures
The synthesis of g-C3N4 requires a thermal polycondensation process that typically occurs at 550°C.
At this temperature, standard laboratory glass would soften or deform. Alumina possesses exceptional heat resistance, maintaining its structural integrity throughout prolonged heating cycles.
Consistency Across Growth Cycles
The material must endure these high temperatures for extended periods to allow for complete polymerization.
Alumina’s stability ensures that the physical dimensions of the reaction vessel do not fluctuate. This provides a consistent environment for the urea precursor to decompose and reorganize into the desired crystalline form.
Preserving Chemical Purity
Inertness Against Reactants
Chemical interactions between a reaction vessel and the reactants are a primary source of failure in material synthesis.
Alumina is chemically inert, meaning it does not react with the urea precursor or the intermediate species generated during heating. This prevents the vessel from eroding or introducing foreign ions into the synthesis.
Preventing Contamination
The goal of this process is to produce high-purity g-C3N4 nanosheets.
By using high-purity alumina, you eliminate the risk of container-derived impurities entering the catalyst material. This is vital because even trace impurities can alter the electronic and physical properties of the final nanosheets.
Controlling the Reaction Environment
Creating a Semi-Closed System
While the material of the crucible is critical, the configuration is equally important. Using a covered alumina crucible creates a semi-closed micro-environment.
This setup prevents the excessive volatilization of reaction intermediates that occurs around 500°C.
Ensuring Yield and Structure
If the intermediates are allowed to escape, the overall yield of g-C3N4 drops significantly.
The semi-closed environment retains these vapors, forcing them to participate in the polymerization. This confinement is essential for ensuring the final powder develops the correct two-dimensional layered structure and light-yellow color indicative of high-quality g-C3N4.
Common Pitfalls to Avoid
The Risk of Open Systems
A common mistake is utilizing an open crucible to allow for easier observation or gas flow.
Doing so disrupts the micro-environment, leading to the rapid loss of precursor material through sublimation. This results in a low yield and potentially incomplete polymerization.
Material Grade Matters
Not all alumina crucibles are created equal; lower-grade ceramics may contain binders or impurities.
You must utilize high-purity alumina to ensure the chemical stability described above. Lower-quality crucibles may degrade under the corrosive nature of the active reaction, similar to how they must resist corrosive metal melts in other high-temperature applications.
Making the Right Choice for Your Goal
To ensure successful synthesis, your equipment choice should align with your specific experimental needs:
- If your primary focus is maximizing yield: Ensure the alumina crucible is paired with a tight-fitting lid to minimize the loss of volatile intermediates.
- If your primary focus is material purity: Verify the specific grade of the alumina to guarantee it is free of trace contaminants that could leach at 550°C.
Summary: The alumina crucible is not just a container; it is an active component of the process control, defining the thermal boundary and chemical purity necessary to successfully engineer g-C3N4 nanosheets.
Summary Table:
| Feature | Requirement for g-C3N4 Synthesis | Benefit of Alumina Crucible |
|---|---|---|
| Thermal Stability | Withstand 550°C calcination | Maintains structural integrity without softening |
| Chemical Inertness | Zero reaction with urea precursors | Prevents impurity leaching and material contamination |
| Environment Control | Semi-closed micro-environment | Retains volatile intermediates to maximize yield |
| Durability | Multiple heating/growth cycles | Long-lasting performance with consistent results |
Optimize Your Material Synthesis with KINTEK
Precision in g-C3N4 nanosheet production starts with the right equipment. Backed by expert R&D and manufacturing, KINTEK offers high-purity alumina crucibles and lab high-temp furnaces—including Muffle, Tube, and Vacuum systems—all customizable for your unique research needs. Ensure maximum yield and chemical purity for your next thermal polycondensation project.
Ready to upgrade your lab’s efficiency? Contact us today to discuss your custom furnace and crucible requirements!
Visual Guide
References
- Guangying Zhou, Jianzhang Fang. Copper-Copper Oxide Heterostructural Nanocrystals Anchored on g-C3N4 Nanosheets for Efficient Visible-Light-Driven Photo-Fenton-like Catalysis. DOI: 10.3390/molecules30010144
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- How do graphite molds in SPS affect maraging steel? Managing Carbon Diffusion for Precise Sintering Results
- Why is a laboratory drying oven or heating plate necessary for Ba7Nb4MoO20? Optimize Slurry Synthesis Results
- Why is a high-purity alumina corundum crucible preferred for melting? Achieve High-Precision Research Integrity
- Why is a molybdenum crucible considered an ideal choice for quartz melting? High-Purity Solutions at 2000°C
- Why is a MgO crucible preferred for VCD? Achieve 3ppm Purity in High-Temperature Metallurgy
- How does a planetary ball mill prepare precursors for furnaces? Unlock Nano-Scale Precision for High-Temp Success
- What is the impact of gas flow meters on catalyst synthesis? Ensure Phase Purity and Precision in (NiZnMg)MoN Production
- Why is the low thermal expansion of quartz important for laboratory applications? Ensure Safety and Precision in High-Heat Experiments



















