Controlled thermal management is essential for processing Ba7Nb4MoO20 precursor slurries effectively. A laboratory drying oven or heating plate is required to remove solvents, such as ethanol, that remain after the ball milling process. This equipment provides a stable temperature environment that facilitates smooth evaporation, preventing the powder from agglomerating due to local overheating and ensuring a fine, loose mixture for the next stage of synthesis.
The Core Takeaway The primary utility of a drying oven or heating plate is the prevention of agglomeration through controlled evaporation. By maintaining a stable thermal environment, you ensure the precursor transforms into a loose, homogeneous powder rather than hard clumps, satisfying the strict physical requirements for successful high-temperature calcination.

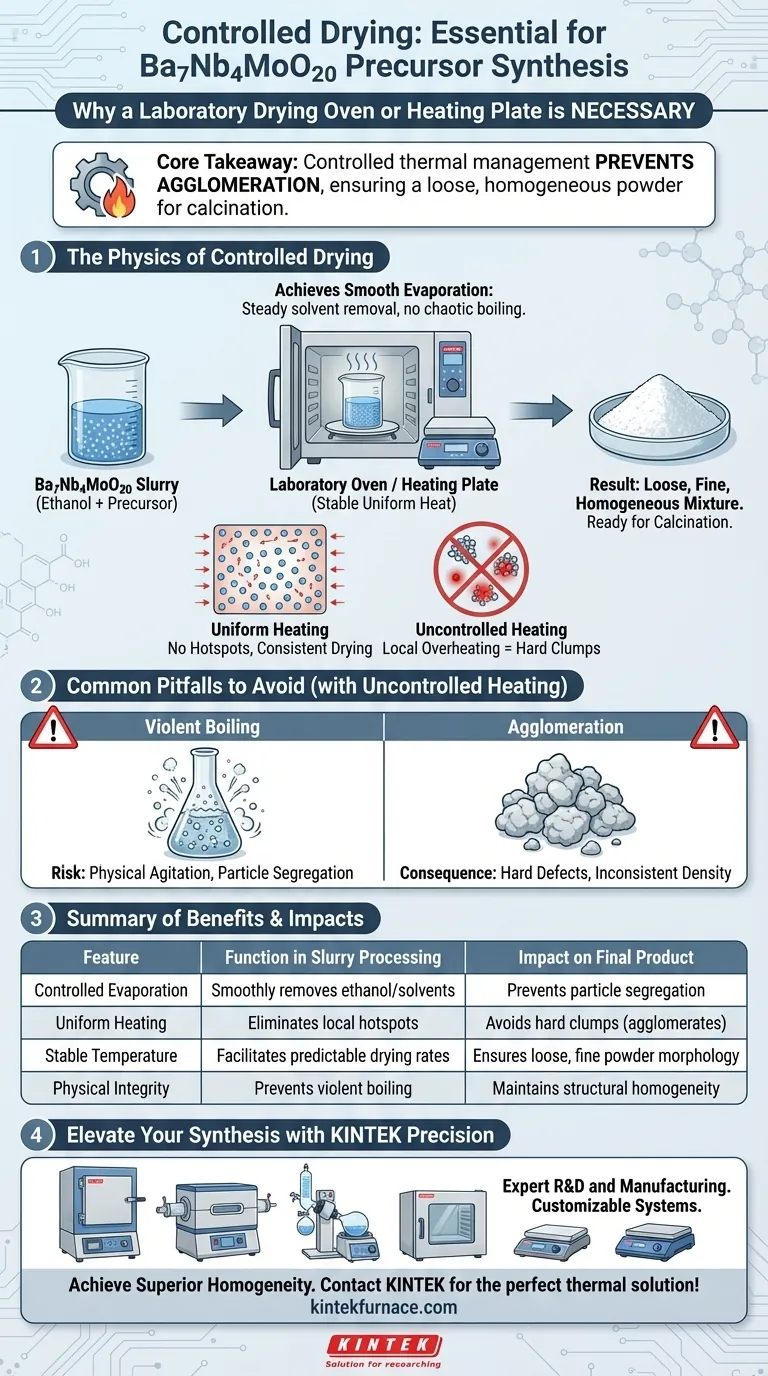
The Physics of Controlled Drying
Achieving Smooth Evaporation
After ball milling, the Ba7Nb4MoO20 slurry is saturated with solvent media, typically ethanol. This solvent must be removed to recover the solid precursor.
A laboratory oven or heating plate provides a uniform heat source. This allows the solvent to evaporate at a steady, predictable rate, rather than boiling off chaotically.
Preventing Local Overheating
Uncontrolled heating sources create "hotspots" within the slurry.
These hotspots cause local overheating, where specific sections of the slurry dry instantly while others remain wet. This disparity forces particles to bind together tightly, creating hard defects in the material structure.
Ensuring a Loose, Fine Mixture
The physical state of the dried precursor dictates the quality of the final product.
By using controlled equipment, you ensure the final result is a loose, fine dry mixture. This morphology is critical because it allows for uniform heat distribution during the subsequent high-temperature calcination process.
Common Pitfalls to Avoid
The Risk of Violent Boiling
While speed is often desired in laboratory settings, rapid drying is detrimental to slurry integrity.
Excessive or unstable heat can cause violent boiling of the solvent. This physical agitation can disrupt the distribution of particles, potentially leading to component segregation or the peeling of coated layers.
The Consequence of Agglomeration
If the drying process is not stable, the powder will form agglomerates (hard clumps).
These clumps prevent the material from reacting uniformly during calcination. Instead of a high-quality ceramic, you risk producing a material with inconsistent density and poor structural properties.
Ensuring Quality in Material Synthesis
To maximize the quality of your Ba7Nb4MoO20 synthesis, apply the following principles:
- If your primary focus is Powder Morphology: Prioritize slow, stable heating to ensure the final mixture remains loose and fine, avoiding hard aggregates.
- If your primary focus is Compositional Homogeneity: Use a drying oven to ensure the solvent is removed evenly from all pores, preventing particle migration or coalescence.
Mastering the drying phase is the invisible step that safeguards the structural integrity of your final ceramic product.
Summary Table:
| Feature | Function in Slurry Processing | Impact on Final Product |
|---|---|---|
| Controlled Evaporation | Smoothly removes ethanol/solvents | Prevents particle segregation |
| Uniform Heating | Eliminates local hotspots | Avoids hard clumps (agglomerates) |
| Stable Temperature | Facilitates predictable drying rates | Ensures loose, fine powder morphology |
| Physical Integrity | Prevents violent boiling | Maintains structural homogeneity |
Elevate Your Material Synthesis with KINTEK Precision
Don't let uncontrolled drying compromise your research outcomes. At KINTEK, we understand that high-performance ceramics like Ba7Nb4MoO20 demand absolute thermal precision. Our laboratory drying ovens and heating plates are designed to provide the stable, uniform environments necessary to prevent agglomeration and ensure perfect powder morphology.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, along with specialized lab heating solutions. All our systems are fully customizable to meet your unique synthesis requirements.
Ready to achieve superior homogeneity in your precursors? Contact us today to find the perfect thermal solution for your lab!
Visual Guide
References
- Bettina Schwaighofer, Ivana Radosavljević Evans. Oxide ion dynamics in hexagonal perovskite mixed conductor Ba<sub>7</sub>Nb<sub>4</sub>MoO<sub>20</sub>: a comprehensive <i>ab initio</i> molecular dynamics study. DOI: 10.1039/d3ma00955f
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Laboratory Muffle Oven Furnace with Bottom Lifting
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- Why are YSZ milling balls selected for mixing Mn2AlB2 precursor powders? Ensure High-Purity MAB Phase Synthesis
- Why are high-purity alumina crucibles used for MAX phase sintering? Ensure Purity in High-Temperature Synthesis
- What are the components of the circulating water vacuum pump and their functions? Discover Oil-Free Vacuum Solutions
- Why are high-performance insulation accessories necessary during the microwave sintering of zirconia ceramics?
- What function does a PTFE liner serve in NiWO4 synthesis? Ensure Purity & Prevent Corrosion in Hydrothermal Reactors
- How do high-precision mass flow controllers (MFC) aid iron oxide reduction studies? Get Accurate Kinetic Data
- How does the use of a high-purity quartz crucible affect silicate inclusions? Master Industrial Melt Simulation
- What role does the planetary ball mill play in LLZO mixing? Unlock High-Performance Solid-State Electrolyte Synthesis



















