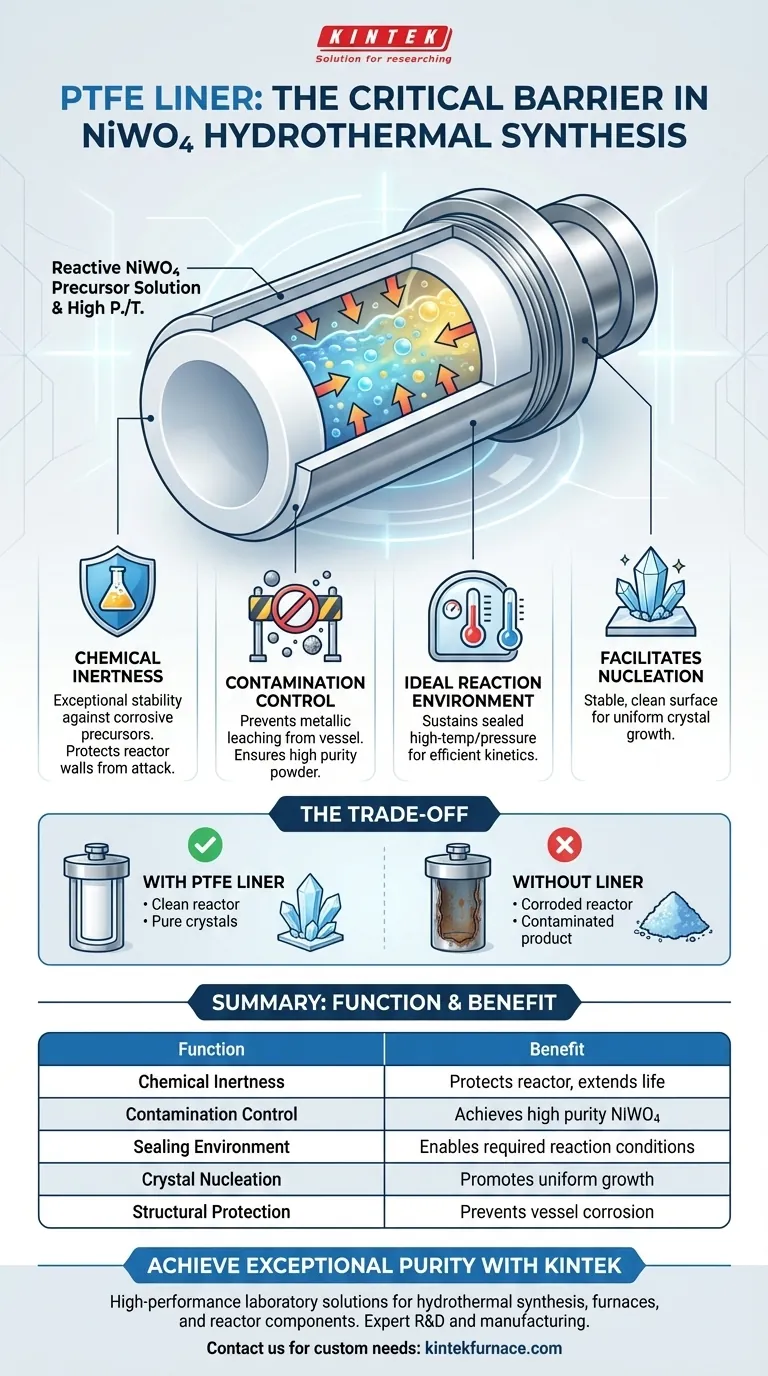
In the hydrothermal synthesis of NiWO4 precursors, the polytetrafluoroethylene (PTFE) liner serves as the critical isolation barrier that ensures the chemical integrity of the reaction. It creates a sealed, high-pressure, and high-temperature environment capable of resisting corrosion from reactive precursor solutions. By separating the chemical mixture from the reactor vessel, it prevents metallic contamination and facilitates the proper crystallization of the material.
The PTFE liner is the primary safeguard against contamination in hydrothermal synthesis. Its core function is to provide a chemically inert environment that resists corrosion, ensuring the final NiWO4 powder retains exceptional purity.

The Role of the Liner in Chemical Synthesis
Ensuring Chemical Inertness
The precursor solutions required to synthesize NiWO4 are chemically active and can be corrosive to standard reactor materials. The PTFE liner provides exceptional chemical stability against these solutions.
It acts as a robust shield, preventing the liquid mixture from chemically attacking the walls of the high-pressure reactor.
Preventing Material Contamination
If the precursor solution were to contact the steel reactor wall directly, impurities would inevitably leach into the mixture.
The PTFE liner strictly isolates the reactants, preventing the introduction of external impurities from the container. This isolation is the deciding factor in achieving high purity in the synthesized powder.
Creating the Ideal Reaction Environment
Sustaining High Pressure and Temperature
Hydrothermal synthesis relies on elevating temperature and pressure to force chemical changes.
The PTFE liner contributes to a sealed environment that can safely sustain these harsh conditions without degrading. This containment is necessary for the reaction kinetics to proceed efficiently.
Facilitating Crystal Nucleation
The synthesis of NiWO4 requires a stable environment for the crystals to form and grow.
By maintaining a clean and chemically stable atmosphere, the liner facilitates the nucleation and growth of NiWO4. It ensures the crystallization process is driven by the precursor chemistry rather than reactions with the vessel surface.
Understanding the Trade-offs
The Necessity of Isolation
While the steel reactor provides the structural strength to hold pressure, it lacks the chemical resistance required for this synthesis.
The trade-off here is clear: utilizing a bare reactor without a liner will result in corrosion of the vessel and contamination of the product. The liner is not optional; it is a requisite component to bridge the gap between structural requirements (steel) and chemical requirements (PTFE).
Ensuring Synthesis Success
To maximize the quality of your NiWO4 precursors, view the liner as an active component of your purity control strategy.
- If your primary focus is High Purity: Prioritize the integrity of the PTFE liner to strictly prevent leaching or impurities from the outer container.
- If your primary focus is Crystal Growth: Rely on the liner's chemical stability to maintain the consistent, unreactive environment necessary for uniform nucleation.
The PTFE liner is the silent guardian of your synthesis, transforming a raw pressure vessel into a precision chemistry tool.
Summary Table:
| Function of PTFE Liner | Benefit to NiWO4 Synthesis |
|---|---|
| Chemical Inertness | Protects reactor from corrosive precursor solutions |
| Contamination Control | Prevents leaching of metallic impurities from vessel walls |
| Sealing Environment | Maintains high-pressure/temp conditions for kinetics |
| Crystal Nucleation | Provides stable, unreactive surface for uniform growth |
| Structural Protection | Extends life of the stainless steel reactor vessel |
Achieve Exceptional Purity in Your Hydrothermal Synthesis
Maximize the integrity of your material research with high-performance laboratory solutions from KINTEK. Whether you are synthesizing NiWO4 precursors or developing advanced nanomaterials, our equipment ensures the chemical stability and precision your work demands.
Backed by expert R&D and manufacturing, KINTEK offers a wide range of lab solutions including Muffle, Tube, Rotary, Vacuum, and CVD systems, along with customizable high-temperature furnaces and reactor components tailored to your unique specifications.
Ready to elevate your lab's efficiency and product quality? Contact us today to discuss your custom furnace and reactor needs!
Visual Guide
References
- Likai Deng, Shifa Wang. Advanced Electrochemical Performance of NiWO4/Graphene Oxide as Cathode Material for Zinc Ion Battery. DOI: 10.3390/en18082023
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- 304 316 Stainless Steel High Vacuum Ball Stop Valve for Vacuum Systems
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
- Laboratory Muffle Oven Furnace with Bottom Lifting
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- What role does a molecular pump set play in an electric current-assisted TLP bonding system? Enhance Vacuum Purity
- What is the technical value of using a narrowband infrared pyrometer? Expert Precision for High-Temp Measurement
- What is the function of high-purity Alumina (Al2O3) crucibles? Enhance Accuracy in Molten Salt Electrochemical Studies
- What is the function of the laboratory furnace? Master Material Transformation with Precision Heating
- What role does a planetary ball mill play in Al-Cr-Cu-Fe-Mn-Ni alloy prep? Master Mechanical Alloying Efficiency
- What is the function of an in-situ heating holder in the study of Peierls transitions in NaRu2O4? Dynamic Lab Insights
- What is the function of an alumina boat during high-temperature activation of porous carbon? Durable Lab Solutions
- Why is it necessary to use high-purity alumina crucibles for sintering hydroxyapatite? Ensure Chemical Phase Purity



















