High-purity Alumina (Al2O3) crucibles function as chemically inert vessels specifically engineered to withstand the rigorous demands of high-temperature electrochemical studies. They serve as a stable containment zone that prevents the molten electrolyte from reacting with the container walls, ensuring the chemical composition remains unaltered during experiments.
The core value of high-purity Alumina lies in its ability to act as a "blank slate." By resisting corrosion and enduring extreme heat, it isolates the molten salt reaction, ensuring that your data reflects the chemistry of the electrolyte rather than contamination from the hardware.

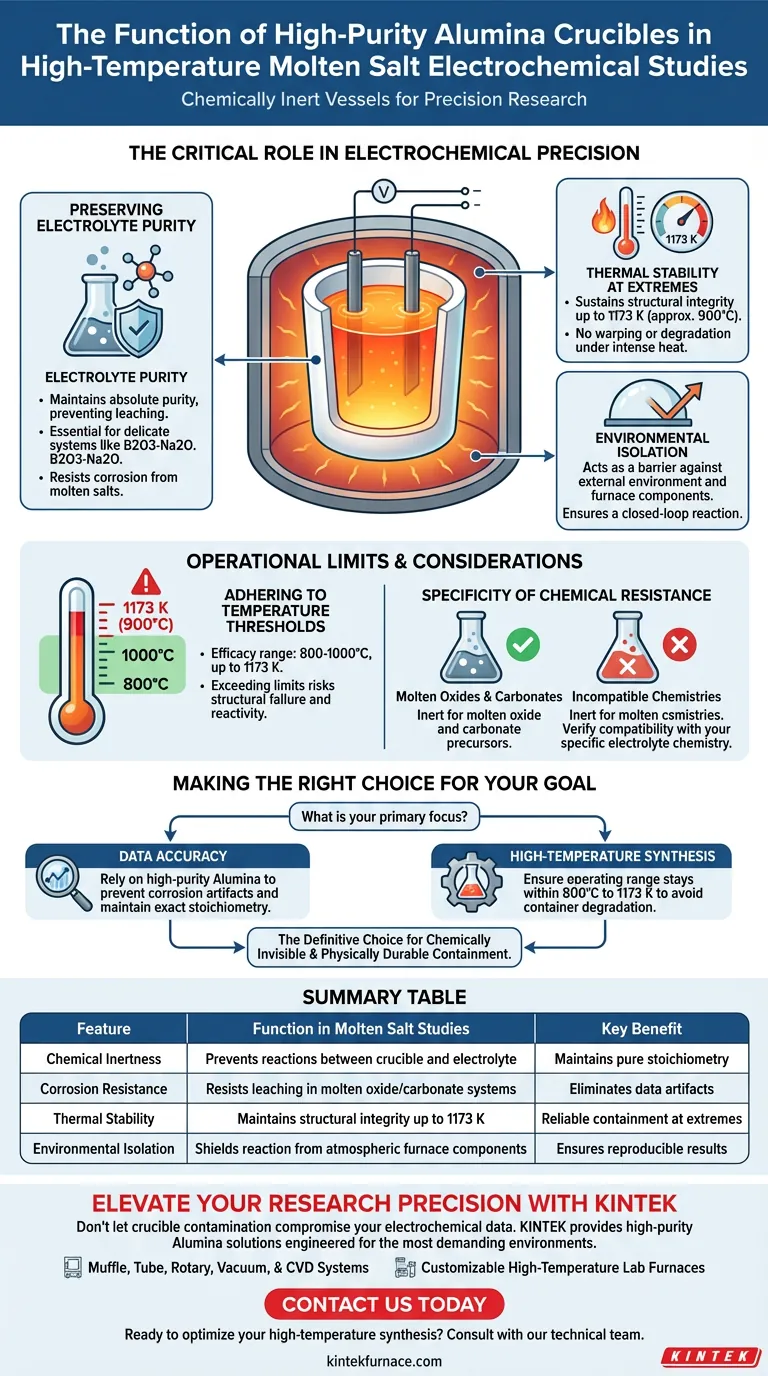
The Critical Role in Electrochemical Precision
Preserving Electrolyte Purity
The primary function of these crucibles is to maintain the absolute purity of the electrolyte system. In delicate studies, such as those involving B2O3-Na2O systems, even trace impurities leached from a container can skew electrochemical results.
High-purity Alumina is highly resistant to corrosion caused by molten salts. This resistance prevents the dissolution of the crucible material into the melt, ensuring the liquid reaction space remains uncontaminated.
Thermal Stability at Extremes
Electrochemical studies often require sustained high temperatures to keep salts in a molten state. Alumina crucibles are designed to maintain structural integrity at temperatures reaching 1173 K.
This thermal stability ensures the crucible does not warp, soften, or degrade during the experiment. It provides a reliable physical boundary for molten oxide electrolysis even under intense thermal stress.
Environmental Isolation
Beyond containing the liquid, the crucible acts as a barrier against the external environment. It prevents the electrolyte from interacting with the atmosphere or furnace components outside the reaction zone.
This isolation is critical for accurate synthesis and analysis. By creating a closed loop for the reaction, the crucible prevents external variables from altering the chemical state of the molten salt.
Operational Limits and Considerations
Adhering to Temperature Thresholds
While Alumina is robust, it is not invincible. The references explicitly highlight efficacy up to 1173 K (approx. 900°C) and general sintering ranges of 800 to 1000 °C.
Exceeding these specific thermal limits can compromise the inertness of the vessel. For experiments requiring significantly higher temperatures than these benchmarks, the protective properties of the Alumina may diminish, risking structural failure or chemical reactivity.
Specificity of Chemical Resistance
The crucible is described as inert specifically for molten oxide environments and carbonate precursors. While excellent for these applications, users must verify that their specific electrolyte chemistry aligns with Alumina's resistance profile.
Making the Right Choice for Your Goal
To ensure the success of your high-temperature electrochemical study, match your experimental needs to the capabilities of the crucible.
- If your primary focus is Data Accuracy: Rely on high-purity Alumina to prevent corrosion artifacts and maintain the exact stoichiometry of electrolytes like B2O3-Na2O.
- If your primary focus is High-Temperature Synthesis: Ensure your operating range stays within the proven stability zone of 800 °C to 1173 K to avoid degrading the container.
High-purity Alumina is the definitive choice for researchers who require a container that is as chemically invisible as it is physically durable.
Summary Table:
| Feature | Function in Molten Salt Studies | Key Benefit |
|---|---|---|
| Chemical Inertness | Prevents reactions between crucible and electrolyte | Maintains pure stoichiometry |
| Corrosion Resistance | Resists leaching in molten oxide/carbonate systems | Eliminates data artifacts |
| Thermal Stability | Maintains structural integrity up to 1173 K | Reliable containment at extremes |
| Environmental Isolation | Shields reaction from atmospheric furnace components | Ensures reproducible results |
Elevate Your Research Precision with KINTEK
Don't let crucible contamination compromise your electrochemical data. KINTEK provides high-purity Alumina solutions engineered to withstand the most demanding molten salt environments. Backed by expert R&D and manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, along with customizable high-temperature lab furnaces designed for your unique experimental needs.
Ready to optimize your high-temperature synthesis? Contact us today to consult with our technical team and find the perfect hardware for your laboratory.
Visual Guide
References
- Joongseok Kim, Kyung‐Woo Yi. Investigation of Low-Temperature Molten Oxide Electrolysis of a Mixture of Hematite and Zinc Oxide. DOI: 10.3390/ma18174116
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
People Also Ask
- What is the significance of using high-purity quartz tubes in MoS2 growth? Ensure High-Purity Crystal Synthesis
- Why is the use of high-purity graphite crucibles essential? Protect TiC-High Manganese Steel During Sintering
- What is the primary purpose of a vacuum pump in photocatalytic CO2 reduction? Ensure Pure Environments for Accurate Data
- What role does a quartz substrate holder play in MoS2 growth? Optimize Thin Film Deposition with Precision Hardware
- Why is a two-stage vacuum unit used in magnesium distillation? For Faster, More Efficient Pumping
- How does a high-precision heating stage contribute to the drying and crystallization of FAPbBr3 nanosheets?
- Why is a graphite crucible preferred for SiNQ synthesis? Master Heat Management in Magnesiothermic Reduction
- What is the role of a laboratory oven in the pre-treatment of Date Palm Stones? Enhance Torrefaction & Grinding Efficiency
