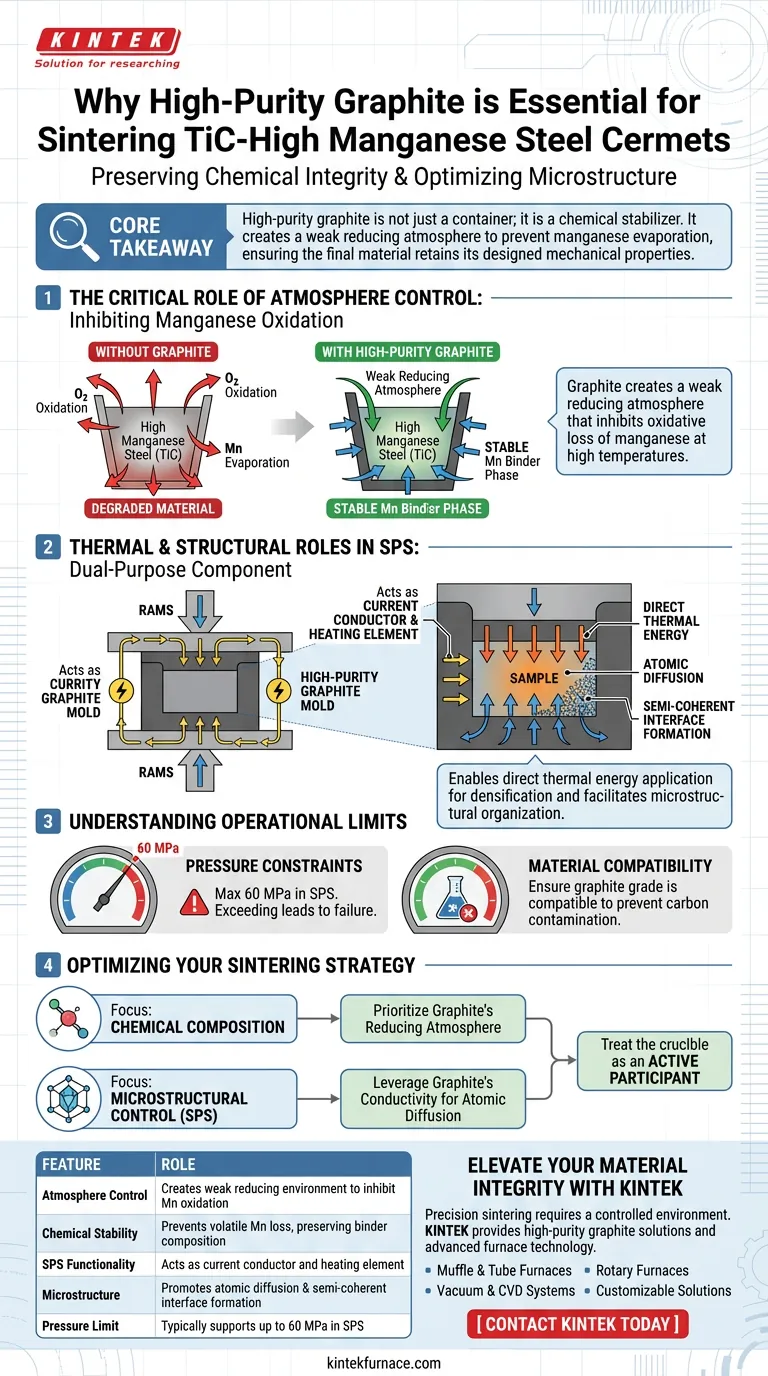
The use of high-purity graphite is strictly required to preserve the chemical integrity of the binder phase within the cermet. Specifically, the graphite creates a weak reducing atmosphere that inhibits the oxidative loss of manganese, a critical element in high manganese steel that would otherwise degrade at sintering temperatures.
Core Takeaway High manganese steel is highly susceptible to oxidation during the sintering process. High-purity graphite acts not just as a container, but as a chemical stabilizer, generating a reducing environment that prevents manganese evaporation and ensures the final material retains its designed mechanical properties.

The Critical Role of Atmosphere Control
Inhibiting Manganese Oxidation
The primary challenge in sintering TiC-high manganese steel cermets is the volatility of manganese. At high sintering temperatures, manganese is prone to rapid oxidative loss.
Creating a Weak Reducing Atmosphere
High-purity graphite addresses this by naturally providing a weak reducing atmosphere surrounding the sample. This chemical environment actively counteracts oxidation, stabilizing the composition of the steel binder.
Guaranteeing Mechanical Performance
The mechanical properties of the final cermet rely heavily on the precise chemical composition of the binder phase. By preventing the loss of manganese, graphite liners ensure the binder performs as intended, maintaining the structural integrity of the cermet.
Thermal and Structural Roles in Spark Plasma Sintering (SPS)
Acting as a Dual-Purpose Component
In advanced processes like Spark Plasma Sintering (SPS), high-purity graphite molds serve a double function. They act as the container for powder shaping while simultaneously serving as the heating element that conducts electric current.
Enabling Direct Thermal Energy Application
Because the graphite mold conducts current, it ensures thermal energy is applied directly to the sample particles. This direct heating promotes atomic diffusion, which is necessary for densification.
Facilitating Microstructural Organization
The thermal environment created by the graphite mold induces the formation of ordered interfaces with semi-coherent characteristics. This specific microstructural organization is essential for minimizing lattice thermal conductivity in the final product.
Understanding the Operational Limits
Pressure Constraints
While high-purity graphite is robust, it has mechanical limits. In SPS applications, these molds generally withstand pressures up to 60 MPa; exceeding this can lead to mold failure or deformation.
Material Compatibility
Graphite is chosen for its high-temperature resistance and chemical stability. However, the user must always ensure that the specific grade of graphite used is compatible with the reactivity of the sample powder to prevent unwanted carbon contamination.
Optimizing Your Sintering Strategy
To achieve the best results with TiC-high manganese steel cermets, align your tooling choices with your specific processing goals:
- If your primary focus is Chemical Composition: Prioritize high-purity graphite explicitly for its ability to generate a reducing atmosphere and prevent manganese depletion.
- If your primary focus is Microstructural Control (SPS): Leverage the graphite mold's conductivity to drive atomic diffusion and create semi-coherent interfaces.
By treating the crucible as an active participant in the chemical process rather than a passive vessel, you ensure the stability and performance of your final cermet product.
Summary Table:
| Feature | Role in TiC-Steel Cermet Sintering |
|---|---|
| Atmosphere Control | Creates a weak reducing environment to inhibit manganese oxidation |
| Chemical Stability | Prevents volatile manganese loss, preserving binder composition |
| SPS Functionality | Acts as both a current conductor and heating element |
| Microstructure | Promotes atomic diffusion and semi-coherent interface formation |
| Pressure Limit | Typically supports up to 60 MPa in SPS applications |
Elevate Your Material Integrity with KINTEK
Precision sintering requires more than just heat; it demands a controlled chemical environment. KINTEK provides high-purity graphite solutions and advanced furnace technology designed to prevent oxidative loss and ensure the mechanical performance of your cermets.
Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of lab high-temp systems, including:
- Muffle & Tube Furnaces for atmosphere-controlled sintering.
- Vacuum & CVD Systems for high-purity material processing.
- Rotary Furnaces and customizable solutions tailored to your unique research needs.
Don't let manganese depletion compromise your results. Contact KINTEK today to discover how our customizable high-temperature solutions can optimize your sintering strategy and safeguard your chemical integrity.
Visual Guide
References
- Nyasha Matsanga, Willie Nheta. An Overview of Thermochemical Reduction Processes for Titanium Production. DOI: 10.3390/min15010017
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Magnesium Extraction and Purification Condensing Tube Furnace
- Chairside Dental Porcelain Zirconia Sintering Furnace with Transformer for Ceramic Restorations
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
People Also Ask
- Why is a benchtop forced air drying oven preferred for microalgae-based nanomaterials? Enhance Powder Quality
- Why are precision molds and laboratory presses critical for niobium-doped TiO2 ceramics? Achieve 94% Theoretical Density
- Why is a covered porcelain crucible used for activated carbon calcination? Secure Your High-Quality Carbon Yield
- Why is a PTFE-lined stainless steel autoclave used for Ni12P5 synthesis? Key Benefits for Nanomaterial Production
- Why is a rotary evaporator used to process separated fractions in hydrotreated coal tar analysis? Enhance Sample Purity
- Why is a high-pressure MFC necessary for CHP systems? Achieve Precision in Catalytic Hydropyrolysis Data
- Why is precise temperature sensor placement critical in high-temp viscometers? Expert Insights for Accurate Melt Data
- Why are alumina crucibles used for CoNb2O6 synthesis? Ensure High-Purity Ceramic Powder Production



















