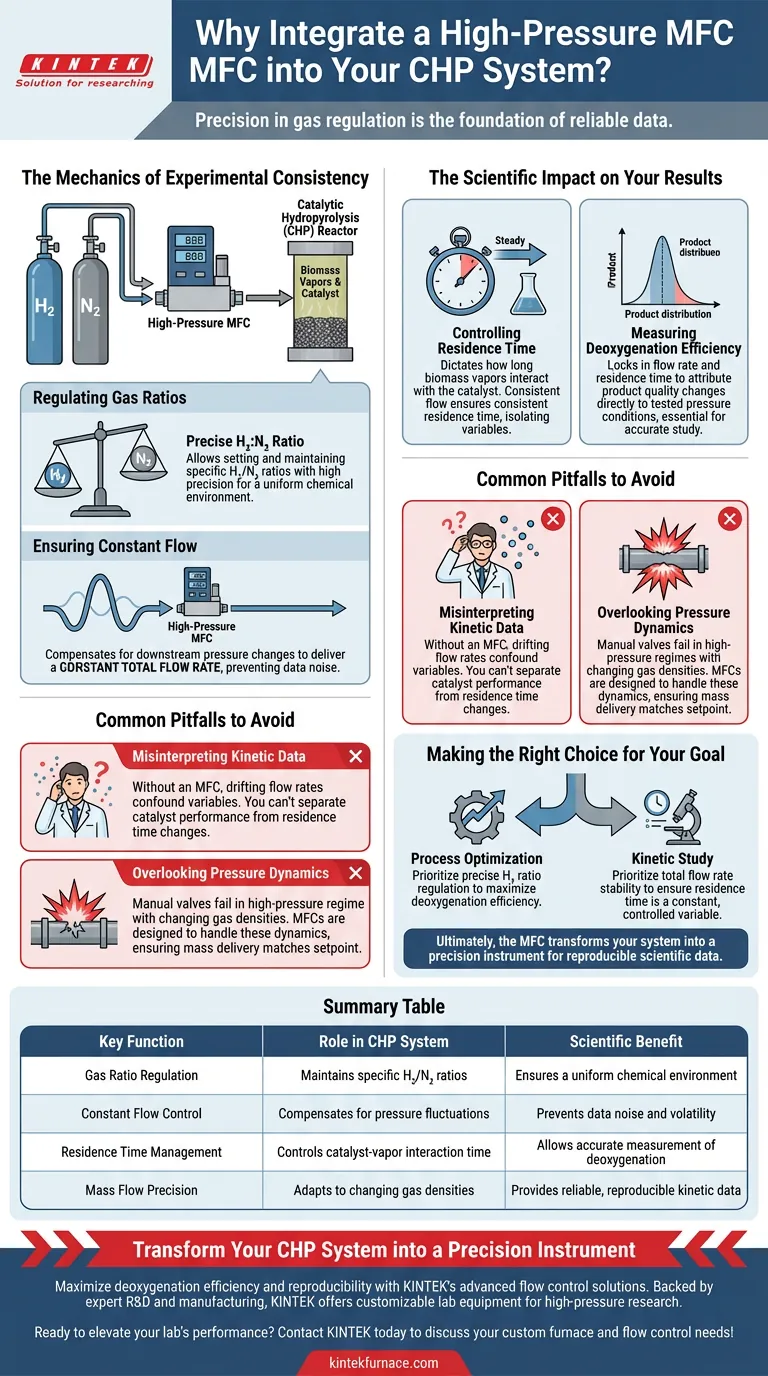
Precision in gas regulation is the foundation of reliable data. In a Catalytic Hydropyrolysis (CHP) system, integrating a high-pressure gas mass flow controller (MFC) is necessary to accurately regulate and maintain specific ratios of hydrogen (H₂) and nitrogen (N₂). This device ensures that both the carrier and reactant gases are delivered at a constant flow rate, stabilizing the fundamental conditions of the experiment.
By maintaining a strict flow rate, the mass flow controller guarantees a consistent residence time within the reactor. This consistency is the only way to accurately isolate variables, allowing you to determine how changes in pressure truly affect deoxygenation efficiency and product distribution.
The Mechanics of Experimental Consistency
Regulating Gas Ratios
In a CHP system, the balance between your reactant gas (H₂) and your inert carrier gas (N₂) is critical.
The MFC allows you to set and maintain this specific ratio with high precision. This ensures that the chemical environment inside the reactor remains uniform throughout the duration of the process.
Ensuring Constant Flow
Experimental conditions in high-pressure systems can be volatile.
The MFC compensates for downstream pressure changes to deliver a constant total flow rate. This stability prevents fluctuations that would otherwise introduce noise into your data set.
The Scientific Impact on Your Results
Controlling Residence Time
The most critical variable controlled by the MFC is residence time.
Residence time dictates how long the biomass vapors interact with the catalyst. If the flow rate varies, the residence time varies, making it impossible to correlate your results to your experimental parameters.
Measuring Deoxygenation Efficiency
A primary goal of CHP is to understand deoxygenation efficiency.
By locking in the flow rate and residence time, you can attribute changes in product quality directly to the pressure conditions you are testing. This isolation of variables is essential for studying product distribution accurately.
Common Pitfalls to Avoid
Misinterpreting Kinetic Data
Without the precision of an MFC, you risk confounding your variables.
If flow rates drift, you cannot determine if a change in yield is due to the catalyst's performance or simply because the reactants spent more or less time in the reaction zone. Reliable kinetic data depends entirely on the flow stability provided by the MFC.
Overlooking Pressure Dynamics
It is a mistake to assume manual valves can handle high-pressure environments effectively.
In high-pressure regimes, gas density changes significantly. An MFC is specifically designed to handle these dynamics, ensuring that the mass of gas delivered matches your setpoint regardless of the system pressure.
Making the Right Choice for Your Goal
To maximize the value of your CHP system, align your MFC usage with your specific research objectives:
- If your primary focus is process optimization: Prioritize the precise regulation of H₂ ratios to maximize deoxygenation efficiency.
- If your primary focus is kinetic study: Prioritize the stability of the total flow rate to ensure residence time remains a constant, controlled variable.
Ultimately, the MFC transforms your system from a simple reactor into a precision instrument capable of generating reproducible scientific data.
Summary Table:
| Key Function | Role in CHP System | Scientific Benefit |
|---|---|---|
| Gas Ratio Regulation | Maintains specific H₂/N₂ ratios | Ensures a uniform chemical environment |
| Constant Flow Control | Compensates for pressure fluctuations | Prevents data noise and volatility |
| Residence Time Management | Controls catalyst-vapor interaction time | Allows accurate measurement of deoxygenation |
| Mass Flow Precision | Adapts to changing gas densities | Provides reliable, reproducible kinetic data |
Transform Your CHP System into a Precision Instrument
Maximize your deoxygenation efficiency and experimental reproducibility with KINTEK’s advanced flow control solutions. Backed by expert R&D and manufacturing, KINTEK offers a wide range of laboratory equipment including Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet the unique demands of your high-pressure research.
Ready to elevate your lab's performance? Contact KINTEK today to discuss your custom furnace and flow control needs!
Visual Guide
References
- Hoda Shafaghat, Olov Öhrman. Customized Atmospheric Catalytic Hydropyrolysis of Biomass to High-Quality Bio-Oil Suitable for Coprocessing in Refining Units. DOI: 10.1021/acs.energyfuels.3c05078
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Kiln for Pyrolysis Plant Heating
- Spark Plasma Sintering SPS Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
People Also Ask
- Why is a platinum-gold alloy crucible utilized during the glass melting process? Achieve Unmatched Purity
- What is the significance of using a quartz boat as a catalyst carrier? Optimize Purity and Kinetics in CCVD
- What is the function of a vacuum pump in tantalum capacitor recycling? Optimize Purity and Speed
- How does a laboratory vacuum pump system contribute to the preparation process of TixNbMoTaW refractory alloys?
- What are the considerations for using high-purity alumina crucibles or boats for SrVO3 sintering? Best Practices
- What are the key characteristics of the alumina furnace tube? Essential for High-Temp Lab Success
- Why is the precision of a Mass Flow Controller (MFC) critical for ethanol vapor detection? Master Accurate Gas Mixing
- What are the technical functions of condensation units and gas collection bags? Optimize Your Reduction Experiments


















