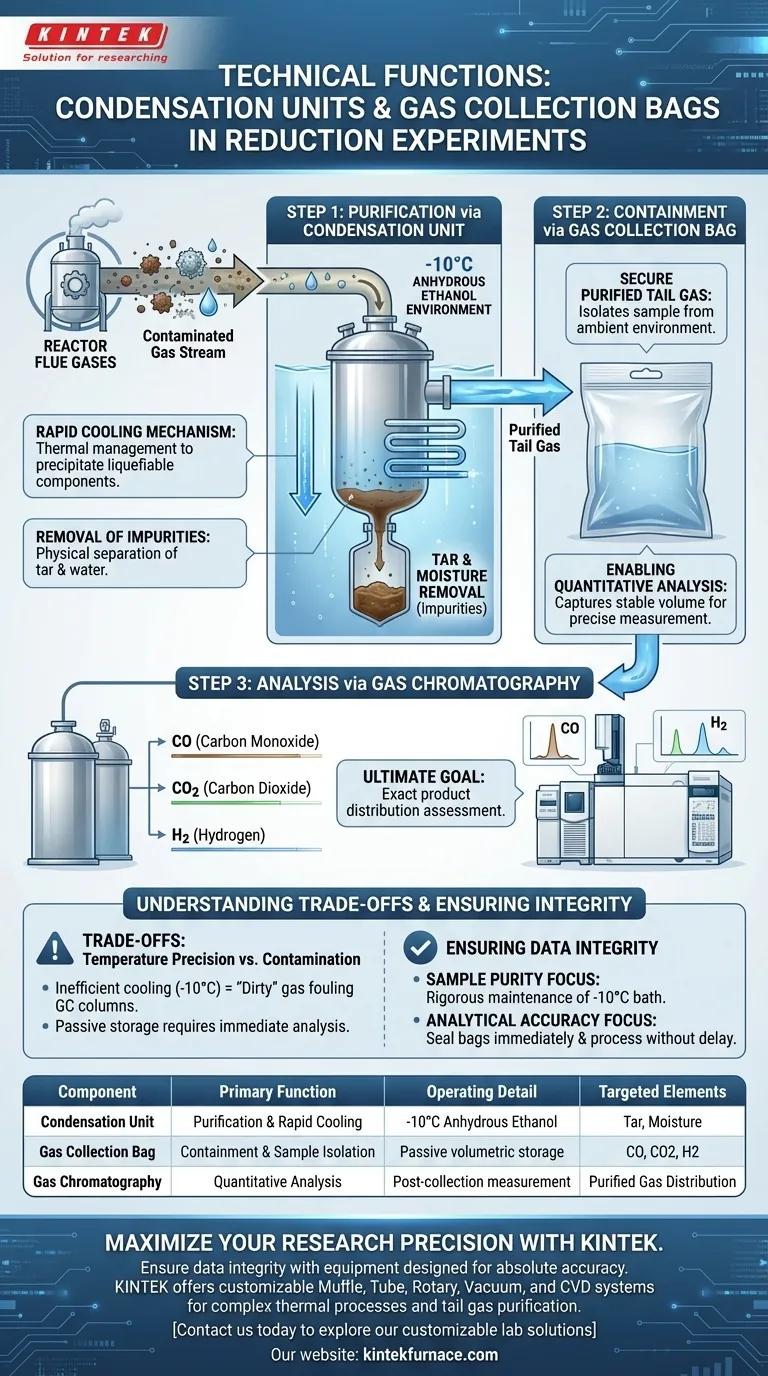
The primary technical functions of these components are purification and containment. During the product collection phase of reduction experiments, condensation units utilizing an anhydrous ethanol environment at -10°C are employed to rapidly cool flue gases, specifically to remove tar and moisture. Following this purification, gas collection bags capture the remaining tail gas to secure it for quantitative analysis via gas chromatography.
The sequence of rapid cooling followed by containment is not merely about storage; it is a quality control measure. By stripping contaminants first, you ensure that the subsequent analysis of CO, CO2, and H2 accurately reflects the reduction process without interference from moisture or tar.

The Role of the Condensation Unit
Rapid Cooling Mechanism
The first critical step in the collection phase is thermal management. The condensation unit utilizes an anhydrous ethanol environment chilled to -10°C. This specific setup is designed to lower the temperature of the reaction flue gases immediately as they exit the reactor.
Removal of Impurities
The technical objective of this rapid cooling is the physical separation of byproducts. By dropping the temperature to -10°C, the system effectively condenses and precipitates tar and moisture out of the gas stream. This ensures that these heavier, liquefiable components do not travel further downstream.
The Function of Gas Collection Bags
Securing Purified Tail Gas
Once the gas stream has been scrubbed of tar and water, it is classified as "purified tail gas." The gas collection bag serves as the containment vessel for this clean sample. It isolates the gas from the ambient environment, preventing dilution or contamination.
Enabling Quantitative Analysis
The ultimate goal of using the collection bag is to facilitate gas chromatography. By capturing a stable volume of the purified gas, researchers can perform a quantitative assessment of the gas product distributions. This allows for the precise measurement of critical reduction gases, specifically CO, CO2, and H2.
Understanding the Trade-offs
Temperature Precision vs. Contamination
The system relies heavily on the specific operating temperature of -10°C. If the temperature is not maintained precisely—or if the anhydrous ethanol environment is compromised—the removal of tar and moisture will be inefficient. This leads to "dirty" gas entering the collection bag, which can foul gas chromatography columns and skew analytical data.
Sample Integrity
While gas bags are effective for short-term storage, they are a passive collection method. They do not actively preserve the sample state; they simply hold it. Therefore, the analysis must occur shortly after collection to prevent any potential diffusion or reaction of the gases within the bag, which would alter the ratios of CO, CO2, and H2.
Ensuring Data Integrity in Your Setup
To maximize the reliability of your reduction experiment data, consider the following regarding your collection system:
- If your primary focus is Sample Purity: rigorous maintenance of the -10°C anhydrous ethanol bath is required to ensure complete removal of tar and moisture before collection.
- If your primary focus is Analytical Accuracy: ensure the gas collection bags are sealed immediately and processed via gas chromatography without delay to obtain exact distributions of CO, CO2, and H2.
This two-step process is the standard for isolating target gases from the complex byproducts of reduction reactions.
Summary Table:
| Component | Primary Technical Function | Operating Detail | Targeted Impurities/Gases |
|---|---|---|---|
| Condensation Unit | Purification & Rapid Cooling | -10°C Anhydrous Ethanol Environment | Tar and Moisture |
| Gas Collection Bag | Containment & Sample Isolation | Passive volumetric storage | CO, CO2, and H2 |
| Gas Chromatography | Quantitative Analysis | Post-collection measurement | Purified Tail Gas Distribution |
Maximize Your Research Precision with KINTEK
Ensure data integrity in your reduction experiments with equipment designed for absolute accuracy. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique laboratory needs. Whether you are managing complex thermal processes or purifying tail gases for analysis, our high-temperature furnaces provide the stability your research demands.
Contact us today to explore our customizable lab solutions
Visual Guide
References
- Menglan Zeng, Fawei Lin. Application of Waste Tire Carbon for Iron-Containing Dust Reduction in Industrial Processes. DOI: 10.3390/app15126504
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- MPCVD Machine System Reactor Bell-jar Resonator for Lab and Diamond Growth
- Cylindrical Resonator MPCVD Machine System for Lab Diamond Growth
People Also Ask
- What is the function of a laboratory electric blast drying oven in biomass pretreatment? Standardize Your Samples
- What is the function of a water-cooled copper crucible? Master High-Purity Alloy Synthesis with KINTEK
- Why is a graphite crucible selected as the high-temperature reaction vessel? Optimize Sodium-Ion Battery Synthesis
- Why are high-alumina crucibles required for static immersion corrosion tests? Ensure Data Purity at 1000°C
- What are the core functions of high-purity graphite molds and graphite paper in SPS? Optimize Sintering Quality
- Why is an alumina crucible necessary for g-C3N4 synthesis? Ensure High Purity & Stability in Polycondensation
- Why are ceramic crucibles required for the high-temperature calcination of dolomite? Ensure High-Purity Results
- What are the technical advantages of using high-purity quartz tubes? Optimize Heat and Purity in Combustion Analysis



