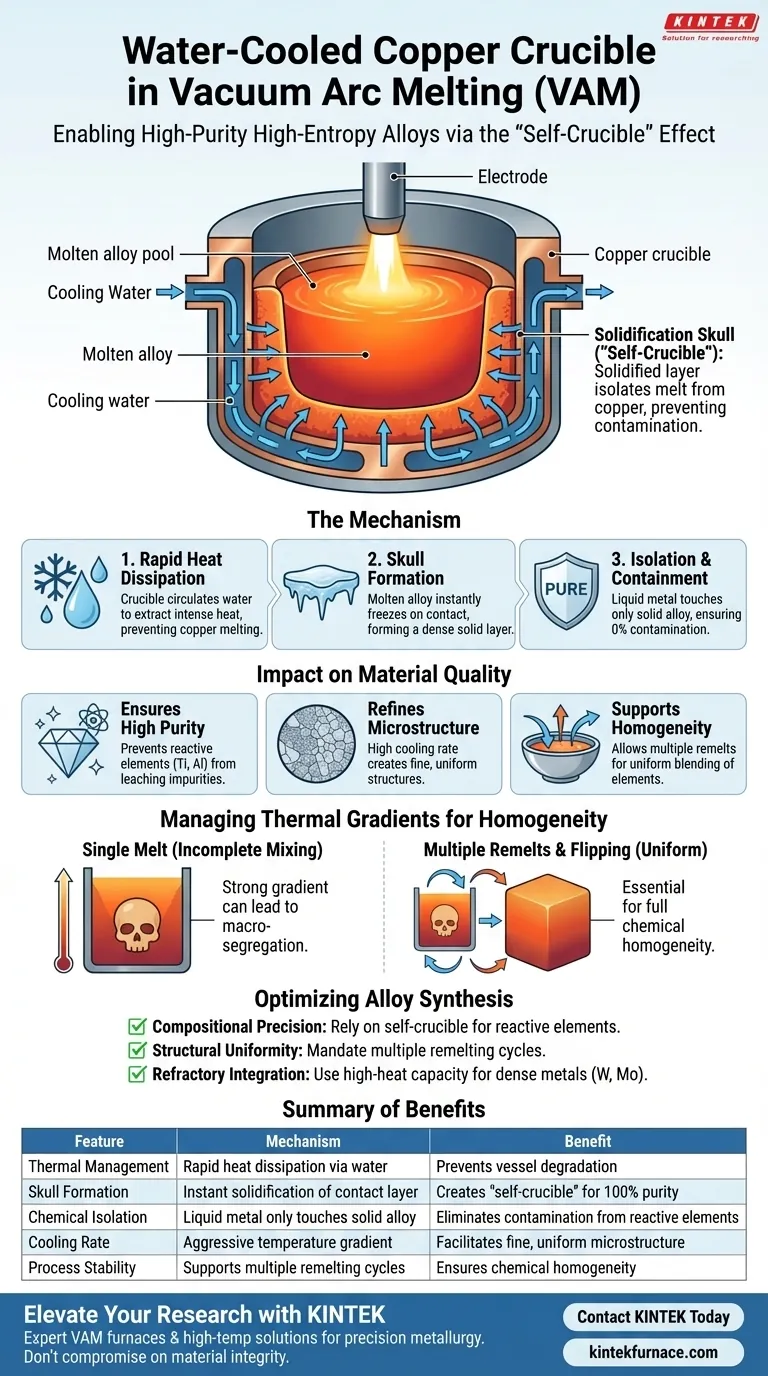
The primary function of a water-cooled copper crucible is to rapidly dissipate heat from the molten alloy, creating a physical barrier known as a "self-crucible." By circulating cooling water, the crucible forces the liquid alloy in contact with its walls to solidify instantly. This solidified layer isolates the high-temperature melt from the copper vessel, preventing chemical reactions and ensuring the purity of the final high-entropy alloy.
The water-cooled crucible acts as a thermal management system that utilizes the alloy's own material to create a protective lining. This allows for the melting of highly reactive or high-melting-point elements without the risk of contamination associated with traditional ceramic crucibles.

The Mechanism of the "Self-Crucible"
Rapid Heat Dissipation
The crucible is engineered to extract heat aggressively using a continuous flow of circulating water. This prevents the copper itself from melting, despite being subjected to the intense heat of the electric arc and the molten alloy.
Formation of the Condensation Layer
When the molten alloy touches the cold crucible wall, it freezes immediately. This forms a dense solidification skull or condensation layer.
Isolation and Containment
This skull layer serves as the actual container for the remaining liquid pool. Because the molten metal only touches solid metal of the same composition—rather than the copper walls—there is no cross-contamination or chemical reaction between the vessel and the alloy.
Impact on Material Quality
Ensuring High Purity
High-entropy alloys often contain reactive elements like aluminum and titanium, or refractory metals like tungsten. The self-crucible layer prevents these elements from leaching impurities from the containment vessel, guaranteeing precise chemical composition for complex alloys like AlCrTiVNbx.
Refining Microstructure
The water-cooled crucible provides an extremely high cooling rate during the process. This rapid solidification facilitates the formation of fine, uniform microstructures within the alloy, which is often superior to the coarser structures formed in slower-cooling environments.
Supporting Homogeneity
While the crucible cools the exterior, the internal arc forces and gravity drive convective mixing within the liquid pool. This environment allows for repeated melting and flipping operations, which are necessary to eliminate macro-segregation and blend elements with vastly different densities.
Understanding the Trade-offs
The Necessity of Multi-Stage Melting
Because the crucible aggressively cools the bottom and sides of the ingot, a strong temperature gradient exists between the liquid top and the solid bottom. This can lead to incomplete mixing in a single pass.
To counter this, the process requires multiple flipping and re-melting operations. This ensures that the material previously trapped in the solid "skull" layer is melted and mixed into the bulk liquid, achieving chemical homogeneity throughout the entire ingot.
Optimizing Your Alloy Synthesis
If your primary focus is Compositional Precision:
- Rely on the self-crucible effect to process reactive elements (like Ti or Al) without fear of crucible-induced contamination.
If your primary focus is Structural Uniformity:
- Mandate multiple remelting cycles to overcome the thermal gradient caused by the water cooling and ensure full convective mixing.
If your primary focus is Refractory Element Integration:
- Utilize the high-heat capacity of the system to melt dense metals (like W or Mo), relying on the protective skull to contain the extreme temperatures required.
The water-cooled copper crucible is the fundamental enabler for processing high-purity, complex alloys that would otherwise destroy standard containment vessels.
Summary Table:
| Feature | Mechanism | Benefit for High-Entropy Alloys |
|---|---|---|
| Thermal Management | Rapid heat dissipation via water circulation | Prevents copper melting and vessel degradation |
| Skull Formation | Instant solidification of contact layer | Creates a "self-crucible" barrier to ensure 100% purity |
| Chemical Isolation | Liquid metal only touches solid alloy | Eliminates contamination from reactive elements (Ti, Al) |
| Cooling Rate | Aggressive temperature gradient | Facilitates fine, uniform microstructure development |
| Process Stability | Supports multiple remelting cycles | Ensures chemical homogeneity in complex alloy compositions |
Elevate Your Materials Research with KINTEK
Precision in high-entropy alloy synthesis starts with superior thermal management. Backed by expert R&D and manufacturing, KINTEK offers high-performance vacuum systems, including specialized Vacuum Arc Melting (VAM) furnaces and customizable CVD/high-temperature systems designed to meet the rigorous demands of modern metallurgy.
Whether you are processing reactive elements or refractory metals, our tailored lab solutions provide the purity and homogeneity your research requires. Don't compromise on material integrity.
Contact KINTEK Today to Customize Your High-Temp Furnace Solution
Visual Guide
References
- Baowei Li, Zhen Peng. Microstructure and Friction Properties of AlCrTiVNbx High-Entropy Alloys via Annealing Manufactured by Vacuum Arc Melting. DOI: 10.3390/ma17040812
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Stainless Steel Quick Release Vacuum Chain Three Section Clamp
- High Performance Vacuum Bellows for Efficient Connection and Stable Vacuum in Systems
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- What is the purpose of using a PID controller to drive a heating furnace? Master Thermal Kinetics Precision
- Why is a copper getter chamber integrated into heating systems? Ensure Ultra-Pure Alloy Processing
- What substances are prohibited from being introduced into the furnace chamber? Prevent Catastrophic Failure
- Why is an external cooling system vital for high-temperature furnace stability? Protect Your Research Integrity
- What customization options are available for alumina ceramic tubes? Tailor for High-Temp, Corrosion-Resistant Applications
- How can the temperature resistance of alumina ceramic furnace tubes be assessed? Ensure Long-Term Reliability in Your Lab
- Why introduce argon flow into a steel crucible for ZK51A alloy? Ensure Safety and High-Purity Melting
- What is the function of ceramic alumina furnace tubes for Ti–Nb–Si alloys? Key Roles in Sintering & Purity



















