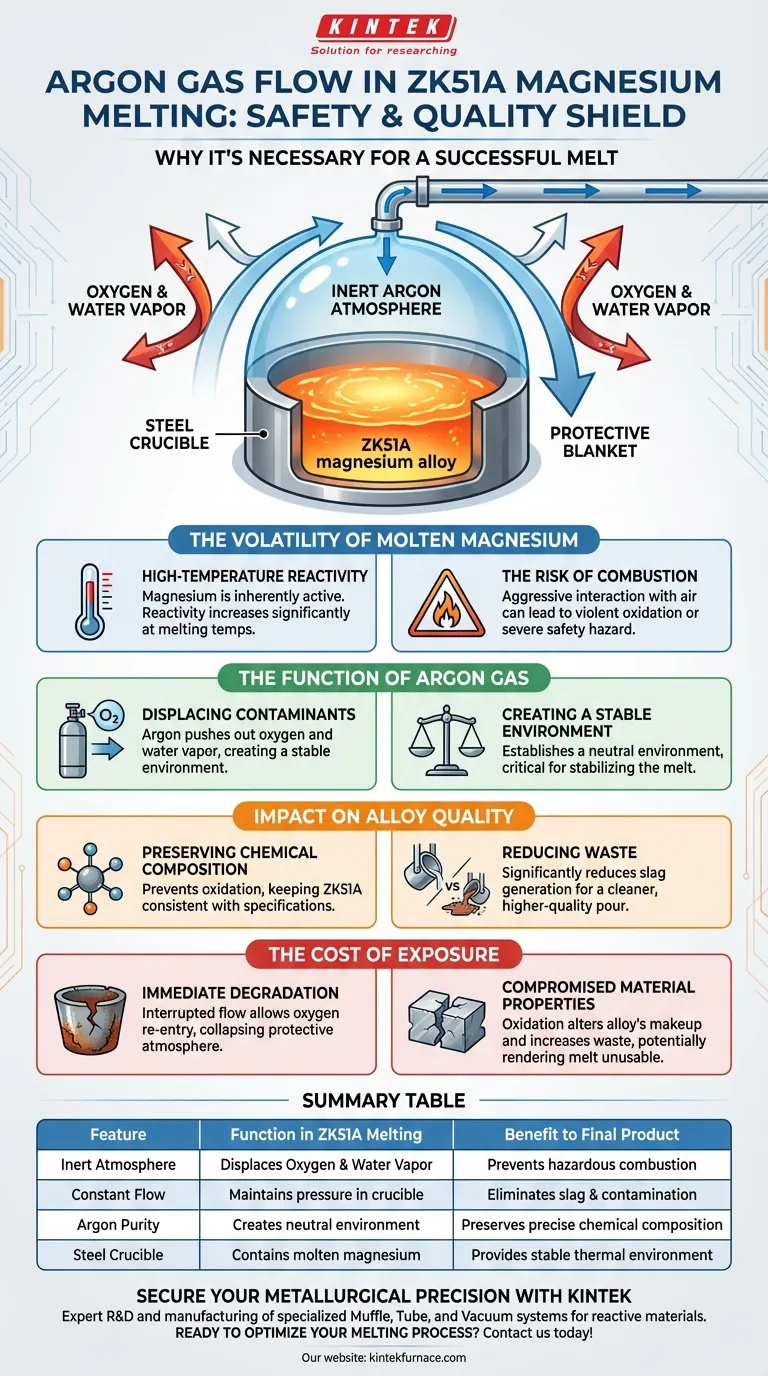
Protection against reactivity is the primary objective. Introducing a constant argon gas flow into the crucible creates an inert protective atmosphere that physically displaces oxygen and water vapor. This prevents the highly reactive ZK51A magnesium alloy from suffering violent oxidation or combustion, while simultaneously preserving the alloy's chemical accuracy.
The introduction of argon is not merely a precaution; it is a fundamental requirement to prevent hazardous combustion and ensure the metallurgical integrity of the final product.
The Volatility of Molten Magnesium
High-Temperature Reactivity
Magnesium is an inherently active metal. When raised to melting temperatures, its reactivity increases significantly.
The Risk of Combustion
Without a protective barrier, molten magnesium interacts aggressively with the surrounding air. This can lead to violent oxidation or even combustion, posing a severe safety hazard to the operation.
The Function of Argon Gas
Displacing Contaminants
Argon acts as a heavy, inert blanket. A constant flow pushes out the reactive elements present in standard air, specifically oxygen and water vapor.
Creating a Stable Environment
By removing these contaminants, argon establishes a neutral environment within the steel crucible. This isolation is critical for stabilizing the melt during the high-temperature phase.
Impact on Alloy Quality
Preserving Chemical Composition
Safety is not the only variable; material science is equally critical. Preventing oxidation ensures the accuracy of the alloy chemical composition, keeping the ZK51A consistent with its specifications.
Reducing Waste
Oxidation produces byproducts that degrade the melt. The use of an argon shield significantly reduces the generation of slag, resulting in a cleaner, higher-quality pour.
The Cost of Exposure
Immediate Degradation
If the gas flow is interrupted or insufficient, the protective atmosphere collapses. This allows oxygen to re-enter the crucible immediately.
Compromised Material Properties
The result of exposure is not just a safety risk, but a metallurgical failure. The resulting oxidation alters the alloy's makeup and increases waste material, rendering the melt potentially unusable.
Making the Right Choice for Your Goal
To achieve a successful melt of ZK51A magnesium alloy, you must prioritize atmosphere control.
- If your primary focus is Safety: Maintain constant flow to prevent contact with oxygen and eliminate the risk of violent combustion.
- If your primary focus is Metallurgical Quality: Use the argon shield to minimize slag formation and strictly maintain the alloy's chemical composition.
A consistent argon flow is the single most effective variable for controlling both the safety and quality of a magnesium melt.
Summary Table:
| Feature | Function in ZK51A Melting | Benefit to Final Product |
|---|---|---|
| Inert Atmosphere | Displaces Oxygen & Water Vapor | Prevents hazardous combustion |
| Constant Flow | Maintains pressure in crucible | Eliminates slag & contamination |
| Argon Purity | Creates neutral environment | Preserves precise chemical composition |
| Steel Crucible | Contains molten magnesium | Provides stable thermal environment |
Secure Your Metallurgical Precision with KINTEK
Don't compromise on safety or material integrity. Backed by expert R&D and manufacturing, KINTEK offers specialized Muffle, Tube, and Vacuum systems designed to handle reactive materials like ZK51A magnesium alloy. Whether you need precise atmosphere control or high-temp durability, our lab furnaces are fully customizable to meet your unique metallurgical needs.
Ready to optimize your melting process? Contact us today to discuss your project!
Visual Guide
References
- Anastasia Akhmadieva, Alexander Vorozhtsov. Structure, Phase Composition, and Mechanical Properties of ZK51A Alloy with AlN Nanoparticles after Heat Treatment. DOI: 10.3390/met14010071
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- How does the vacuum pumping principle of a circulating water vacuum pump differ from jet pumping? Compare Mechanisms & Uses
- What role does a high-precision constant temperature drying oven play in battery electrode preparation? Master Battery Performance
- What are the benefits of the improved circulating water vacuum pump? Save Costs and Go Green in Your Lab
- What are the advantages of 0.7 mm quartz capillaries for SXRD? Optimize High-Energy In-Situ X-ray Experiments
- How does the gas control system regulate the plasma nitriding process? Master Your N2/H2 Mixture for Superior Surfaces
- Why is the integration of a K-type thermocouple and a data logger necessary for Vanadis 60 steel? Unlock Precision.
- What are the considerations for using high-purity alumina crucibles or boats for SrVO3 sintering? Best Practices
- What is the significance of using a laboratory electric thermostatic blast drying oven for biomass briquette moisture control?



















