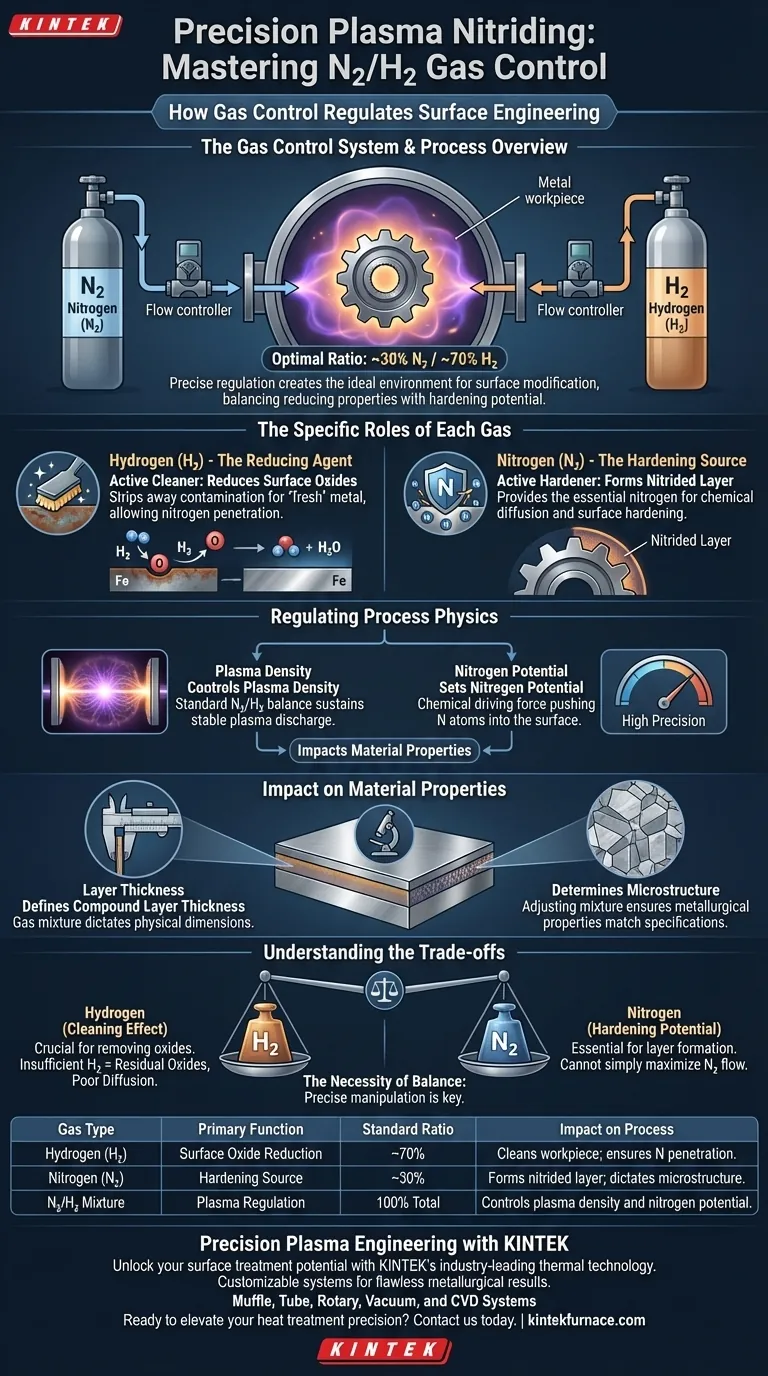
The gas control system in plasma nitriding functions by precisely metering the flow and ratio of nitrogen (N2) and hydrogen (H2) into the vacuum chamber. This regulation typically targets a specific mixture, such as 30% nitrogen and 70% hydrogen, to create the optimal environment for surface modification.
By balancing the reducing properties of hydrogen with the hardening potential of nitrogen, the system enables fine control over plasma density and nitrogen potential, directly dictating the thickness and quality of the final compound layer.

The Specific Roles of Each Gas
Hydrogen as a Reducing Agent
Hydrogen (H2) acts as the active cleaner in the process. Its primary function is to reduce surface oxides that naturally exist on the workpiece.
By stripping away these oxides, hydrogen ensures the metal surface is chemically "fresh." This preparation is critical for allowing nitrogen to penetrate the material effectively.
Nitrogen as the Hardening Source
Nitrogen (N2) is the active hardening ingredient. It serves as the direct source for the nitrided layer that forms on the component.
Without a precise supply of nitrogen, the chemical diffusion required to harden the surface cannot occur.
Regulating Process Physics
Controlling Plasma Density
The gas control system uses the N2/H2 ratio to manipulate the physical environment within the chamber. Changing the mixture directly affects the plasma density.
A common operational baseline is a mixture of 30% Nitrogen and 70% Hydrogen. Maintaining this specific balance is necessary to sustain a stable plasma discharge suitable for treatment.
Managing Nitrogen Potential
Beyond density, the gas ratio establishes the nitrogen potential. This variable determines the chemical driving force that pushes nitrogen atoms into the steel surface.
High precision in the gas control system allows operators to dial in the exact potential required for the specific alloy being treated.
Impact on Material Properties
Defining Layer Thickness
The regulation of these gases is the primary lever for controlling the physical dimensions of the treatment. The specific gas mixture dictates the thickness of the compound layer.
Determining Microstructure
The gas ratio does not just affect how deep the layer goes, but how it forms. Precise control allows for the adjustment of the microstructure of the iron-nitrogen compound.
This capability ensures that the final metallurgical properties match the engineering specifications of the part.
Understanding the Trade-offs
The Necessity of Balance
While nitrogen is required for hardening, you cannot simply maximize nitrogen flow. If the hydrogen ratio is too low, the cleaning effect is compromised.
The Risk of Residual Oxides
Insufficient hydrogen leads to unreduced surface oxides. These oxides act as a barrier, preventing uniform nitrogen diffusion and resulting in an inconsistent or defective compound layer.
Making the Right Choice for Your Goal
To apply this to your specific process, consider the following operational priorities:
- If your primary focus is surface activation: Prioritize maintaining a sufficient hydrogen ratio (often nearly 70%) to ensure complete reduction of surface oxides.
- If your primary focus is layer specification: Finely tune the nitrogen flow to adjust the nitrogen potential, which will directly alter the thickness and microstructure of the compound layer.
Precise manipulation of the N2/H2 ratio is the defining factor in transitioning from a simple heat treatment to high-precision surface engineering.
Summary Table:
| Gas Type | Primary Function | Standard Ratio | Impact on Process |
|---|---|---|---|
| Hydrogen (H2) | Surface Oxide Reduction | ~70% | Cleans workpiece; ensures nitrogen penetration. |
| Nitrogen (N2) | Hardening Source | ~30% | Forms the nitrided layer; dictates microstructure. |
| N2/H2 Mixture | Plasma Regulation | 100% Total | Controls plasma density and nitrogen potential. |
Precision Plasma Engineering with KINTEK
Unlock the full potential of your surface treatments with KINTEK’s industry-leading thermal technology. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, along with other lab high-temp furnaces—all fully customizable to meet your unique gas regulation needs.
Whether you are refining the N2/H2 balance for aerospace components or scaling up industrial nitriding, our systems provide the stability and control required for flawless metallurgical results.
Ready to elevate your heat treatment precision? Contact us today to discuss your custom furnace solution with our engineering team.
Visual Guide
References
- İsmail Aykut Karamanlı, Okan Ünal. Study of the Wear Resistance Plasma Nitrided GGG60 by Optimization of Surface Treatment Conditions Using Response Surface Methodology. DOI: 10.1007/s40962-024-01310-y
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- What role does a laboratory vacuum pump play in a static batch desulfurization evaluation system? Ensure Data Integrity
- What are the specific functions of a magnetic stirrer and a condenser reflux apparatus in the synthesis of KCC-1? Expert Insights
- Why is a laboratory vacuum drying oven utilized for recovered carbon black? Preserve rCB Integrity and Pore Structure
- What role does a laboratory hydraulic press play in manufacturing nickel composites? Achieving Maximum Density
- Why is a glassy carbon boat preferred over an alumina crucible for Na3Cu4Se4? Ensuring Phase Purity in Flux Synthesis
- What types of trays are compatible with MoSi2 heating elements? Ensure Optimal Performance and Longevity
- Why is a vacuum pump utilized in research concerning the reaction of magnesium with carbon dioxide and nitrogen? Ensure Data Integrity
- How has the circulating water vacuum pump been received in practical use? Durable, Cost-Effective for Lab Tasks



















