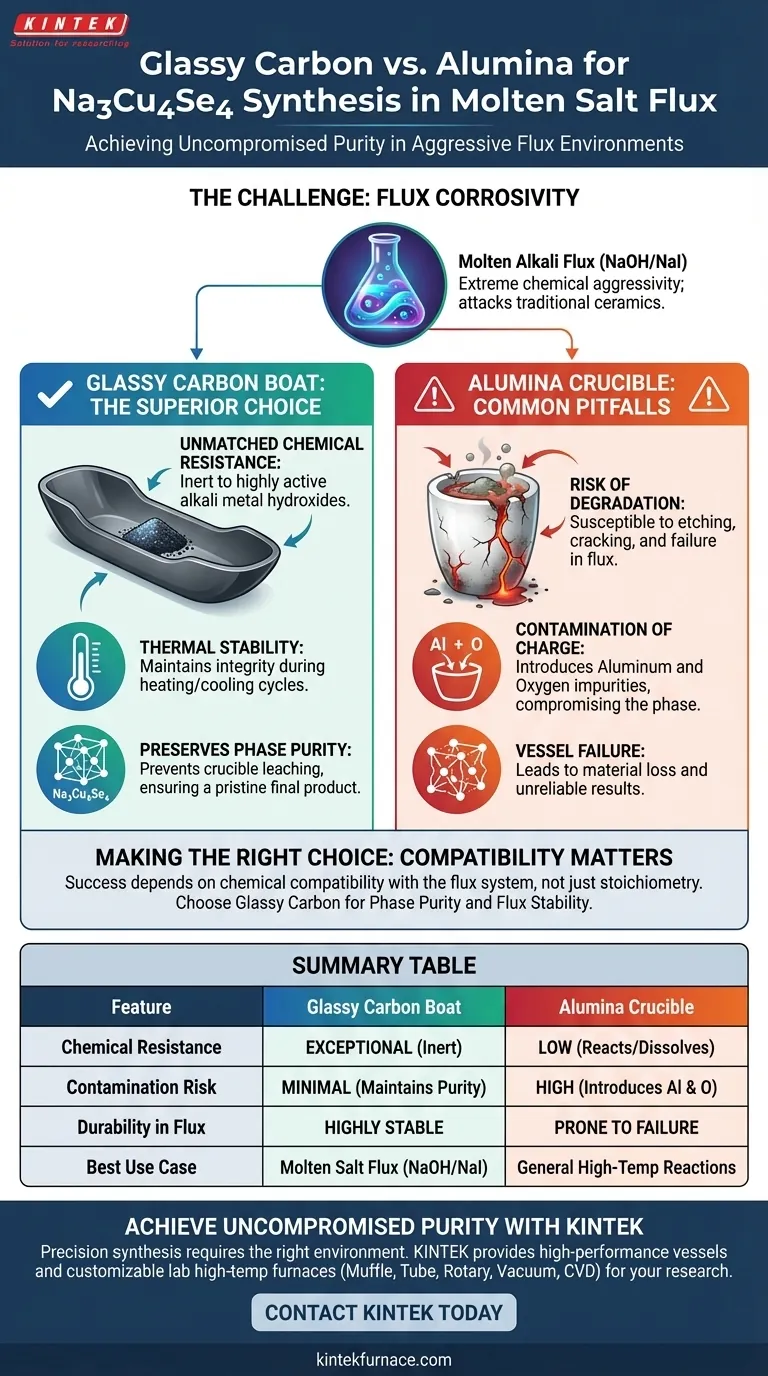
The preference for a glassy carbon boat over standard alumina crucibles is dictated by the extreme chemical corrosivity of the alkali metal hydroxide-sodium iodide flux used in this synthesis. While alumina is sufficient for many reactions, it degrades rapidly when exposed to this specific highly active molten salt mixture. Glassy carbon provides the necessary chemical inertness to prevent the vessel from reacting with the flux and contaminating the final product.
The synthesis of Na3Cu4Se4 relies on maintaining a chemically pristine environment amidst a highly aggressive flux. Glassy carbon is the critical enabler, offering superior chemical resistance that prevents crucible degradation and ensures the phase purity of the final material.

The Challenge of Flux Corrosivity
Understanding the Reaction Environment
The synthesis of the Na3Cu4Se4 phase utilizes a specific alkali metal hydroxide-sodium iodide mixed molten salt flux.
This mixture creates a highly active chemical environment that is far more aggressive than standard solid-state reactions.
The Vulnerability of Ceramics
Traditional ceramic materials, such as alumina (aluminum oxide) or porcelain, are generally susceptible to attack by strong alkali fluxes.
When these crucibles are exposed to the molten hydroxide mixture, the container walls begin to dissolve or react chemically with the flux.
Why Glassy Carbon is Superior
Unmatched Chemical Resistance
Glassy carbon is distinct from standard ceramics because it possesses superior chemical resistance to corrosive salts.
It remains inert even when in direct contact with the highly active alkali metal hydroxide flux.
Thermal Stability
In addition to chemical inertness, glassy carbon offers excellent thermal stability at the temperatures required for this synthesis.
This ensures the boat maintains its structural integrity throughout the heating and cooling cycles of the flux method.
Preserving Phase Purity
The ultimate goal of using glassy carbon is to protect the integrity of the Na3Cu4Se4 phase.
By using a material that does not leach into the melt, you ensure the final product remains free from impurities derived from the container.
Common Pitfalls to Avoid
The Risk of Crucible Degradation
Attempting this synthesis in an alumina or porcelain crucible is a common error that leads to vessel failure.
The corrosive flux will etch the crucible, potentially causing it to crack or leak during the procedure.
Contamination of the Charge
The most significant downside of using the wrong vessel is chemical contamination.
As an alumina crucible degrades, aluminum and oxygen atoms are introduced into the molten flux, compromising the purity of the charge-unbalanced Na3Cu4Se4 phase.
Making the Right Choice for Your Goal
Selecting the correct reaction vessel is not a matter of cost, but of chemical compatibility with your specific flux system.
- If your primary focus is Phase Purity: Use glassy carbon to ensure no foreign elements leach from the crucible into your crystal lattice.
- If your primary focus is Flux Stability: Avoid oxide-based ceramics (like alumina) whenever working with aggressive alkali metal hydroxide fluxes to prevent reaction leaks.
The success of a molten salt synthesis often depends as much on the inertness of the container as it does on the stoichiometry of the reagents.
Summary Table:
| Feature | Glassy Carbon Boat | Alumina Crucible |
|---|---|---|
| Chemical Resistance | Exceptional; inert to alkali hydroxides | Low; reacts with and dissolves in flux |
| Contamination Risk | Minimal; maintains phase purity | High; introduces Al and O impurities |
| Durability in Flux | Highly stable and long-lasting | Prone to etching, cracking, and leaking |
| Best Use Case | Molten salt flux (NaOH/NaI) systems | General high-temp solid-state reactions |
Achieve Uncompromised Material Purity with KINTEK
Precision in material synthesis starts with the right environment. Whether you are performing complex molten salt flux reactions or high-temperature sintering, KINTEK provides the high-performance vessels and equipment your research demands. Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, along with specialized lab high-temp furnaces—all fully customizable to meet your unique chemical compatibility and thermal requirements.
Don't let crucible degradation compromise your results. Contact KINTEK today to discuss your specific needs with our technical team and discover how our advanced lab solutions can enhance your lab's efficiency and ensure the success of your most challenging synthesis projects.
Visual Guide
References
- С.А. Новиков, Vladislav V. Klepov. Structural evolution and bonding features of electron deficient copper chalcogenides. DOI: 10.1039/d5ce00479a
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What is the purpose of configuring a hot gas filter within a Catalytic Hydropyrolysis (CHP) process? Ensure Reactor Life
- What are the advantages of nickel crucibles for KOH activation? Ensure High Purity & Thermal Stability up to 700°C
- What is the purpose of a water-cooling jacket in a methane cracking reactor? Prevent Blockages & Thermal Damage
- Why is laboratory heating equipment critical for photothermal actuators? Master Structural Curing & Precision Thermal Control
- Why must alloy samples be sealed in vacuum-evacuated fused silica containers during diffusion annealing processes?
- Why is a vacuum drying oven essential for Pd-Ni/ZrO2 catalyst preparation? Ensure Uniform Metal Distribution
- What are the placement requirements for high-precision standard thermocouples? Master Sensor Calibration Accuracy
- What is the primary function of a high-alumina powder crucible? Ensure Purity in Maraging Steel Pre-treatment



















