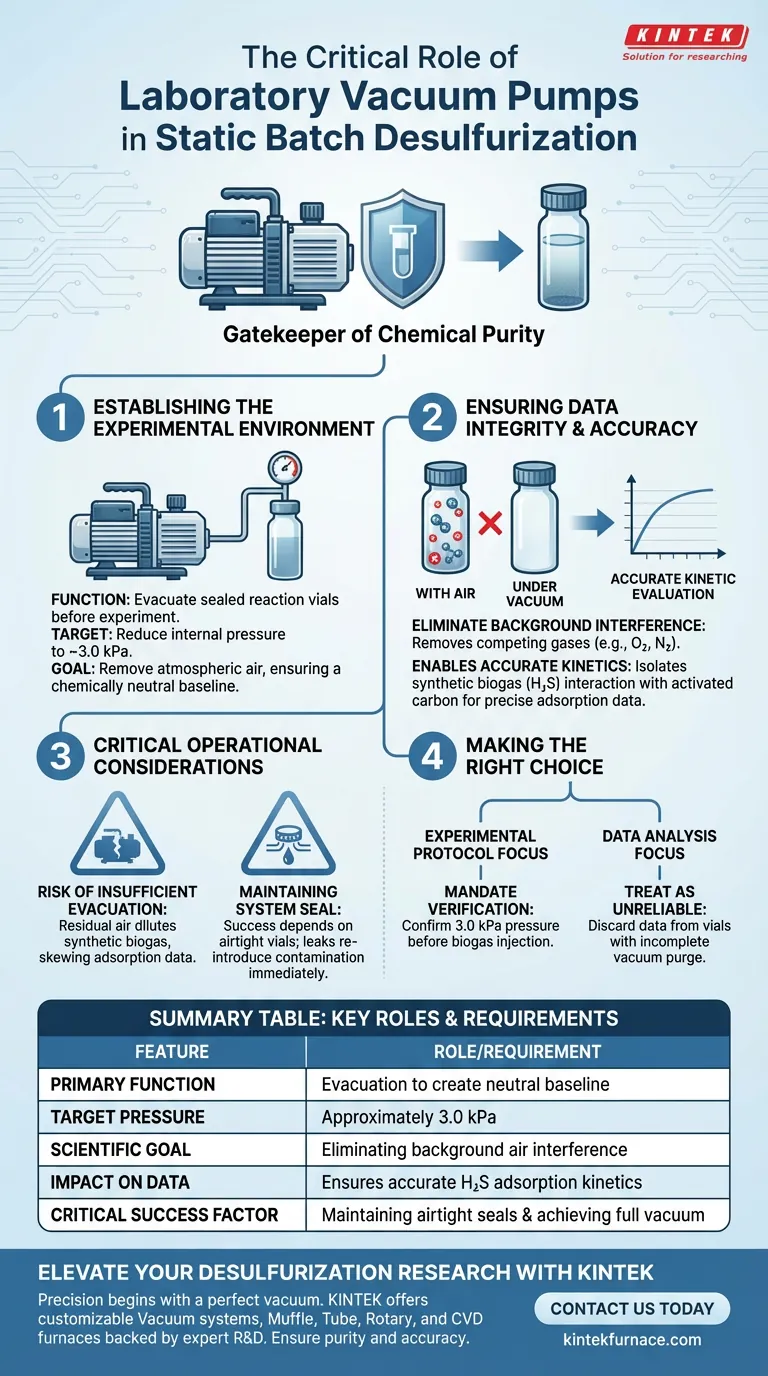
The laboratory vacuum pump serves as a critical preparation tool for establishing a controlled experimental baseline. Its primary function is to evacuate sealed reaction vials before the experiment begins, reducing the internal pressure to approximately 3.0 kPa. This step is mandatory to remove atmospheric air, ensuring that the environment is chemically neutral before the introduction of synthetic biogas.
Accuracy in static batch desulfurization relies on the vacuum pump to eliminate background air interference, ensuring that subsequent measurements of hydrogen sulfide adsorption reflect only the interaction between the biogas and the activated carbon.

Establishing the Experimental Environment
Evacuation of Reaction Vials
The vacuum pump interacts directly with the sealed reaction vials prior to the start of the reaction.
Its objective is to physically extract the existing volume of air trapped within the closed system. This prevents the "starting" atmosphere from being a random variable in the experiment.
Achieving Specific Pressure Targets
The pump must be capable of reducing the internal system pressure to a precise target of approximately 3.0 kPa.
Reaching this specific low-pressure threshold is the indicator that the vial is sufficiently evacuated and ready for the next stage of the process.
Ensuring Data Integrity and Accuracy
Eliminating Background Interference
The primary scientific reason for using the vacuum pump is to remove background air interference.
If atmospheric air remains in the vial, its components (such as nitrogen or oxygen) could compete with the target gases or alter the partial pressures within the system.
Enabling Accurate Kinetic Evaluation
By creating a vacuum, the system ensures that the subsequently injected synthetic biogas is the only gas interacting with the activated carbon.
This isolation allows researchers to accurately evaluate the hydrogen sulfide (H2S) adsorption kinetics. Without this step, kinetic data would be corrupted by the presence of non-target atmospheric gases.
Critical Operational Considerations
The Risk of Insufficient Evacuation
If the vacuum pump fails to reach the 3.0 kPa target, residual air remains in the vial.
This residual air dilutes the synthetic biogas, leading to skewed adsorption data and potentially invalidating the kinetic analysis of the activated carbon.
Maintaining System Seal
The effectiveness of the vacuum pump is entirely dependent on the integrity of the reaction vial seals.
Even a high-performance pump cannot compensate for a leaking vial, which will re-introduce background interference immediately after evacuation stops.
Making the Right Choice for Your Goal
To ensure valid results in your desulfurization evaluation, apply the following based on your specific focus:
- If your primary focus is Experimental Protocol: Mandate a verification step to confirm internal pressure drops to 3.0 kPa before injecting any synthetic biogas.
- If your primary focus is Data Analysis: Treat any data collected from vials that did not undergo a complete vacuum purge as unreliable due to atmospheric contamination.
The vacuum pump is not merely a utility; it is the gatekeeper of the experiment's chemical purity.
Summary Table:
| Feature | Role/Requirement |
|---|---|
| Primary Function | Evacuation of reaction vials to create a neutral baseline |
| Target Pressure | Approximately 3.0 kPa |
| Scientific Goal | Eliminating background air interference (Oxygen/Nitrogen) |
| Impact on Data | Ensures accurate measurement of H2S adsorption kinetics |
| Critical Success Factor | Maintaining airtight system seals and achieving full vacuum |
Elevate Your Desulfurization Research with KINTEK
Precision in static batch desulfurization begins with a perfect vacuum. At KINTEK, we understand that background interference can compromise your kinetic data. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of lab solutions, including Vacuum systems, Muffle, Tube, Rotary, and CVD furnaces, all customizable to meet your unique experimental needs.
Ready to ensure the purity of your experimental environment? Contact us today to discover how our high-performance laboratory equipment can deliver the accuracy your research demands.
Visual Guide
References
- Mayerlin Edith Acunã Montaño, Alesandro Bail. Performance assessment of activated carbon thermally modified with iron in the desulfurization of biogas in a static batch system supported by headspace gas chromatography. DOI: 10.1186/s40543-024-00432-6
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What is the role of quartz capillaries in the vacuum sealing process of sulfur? Enhance Purity and In-Situ Analysis
- Why is a heating magnetic stirrer used for the acid activation of zeolites? Precision in Thermal & Kinetic Control
- Why is a graphite thermal baffle necessary for thermal field control? Master Single-Crystal Growth Quality
- Why are high-purity alumina tubes and crucibles preferred for high-temperature smelting? Ensure Maximum Sample Purity
- How does the use of Matched Thermal Baffles (MTB) benefit directional solidification? Achieve Superior Crystal Integrity
- Why use a PLC and touch screen for magnesium vacuum distillation? For Superior Control and Safety
- What roles do high-purity graphite molds perform in A357 sintering? Enhancing Aluminum Matrix Composite Performance
- How does a heating stage contribute to the quality of multi-material 3D printing? Optimize Precision and Stability



















