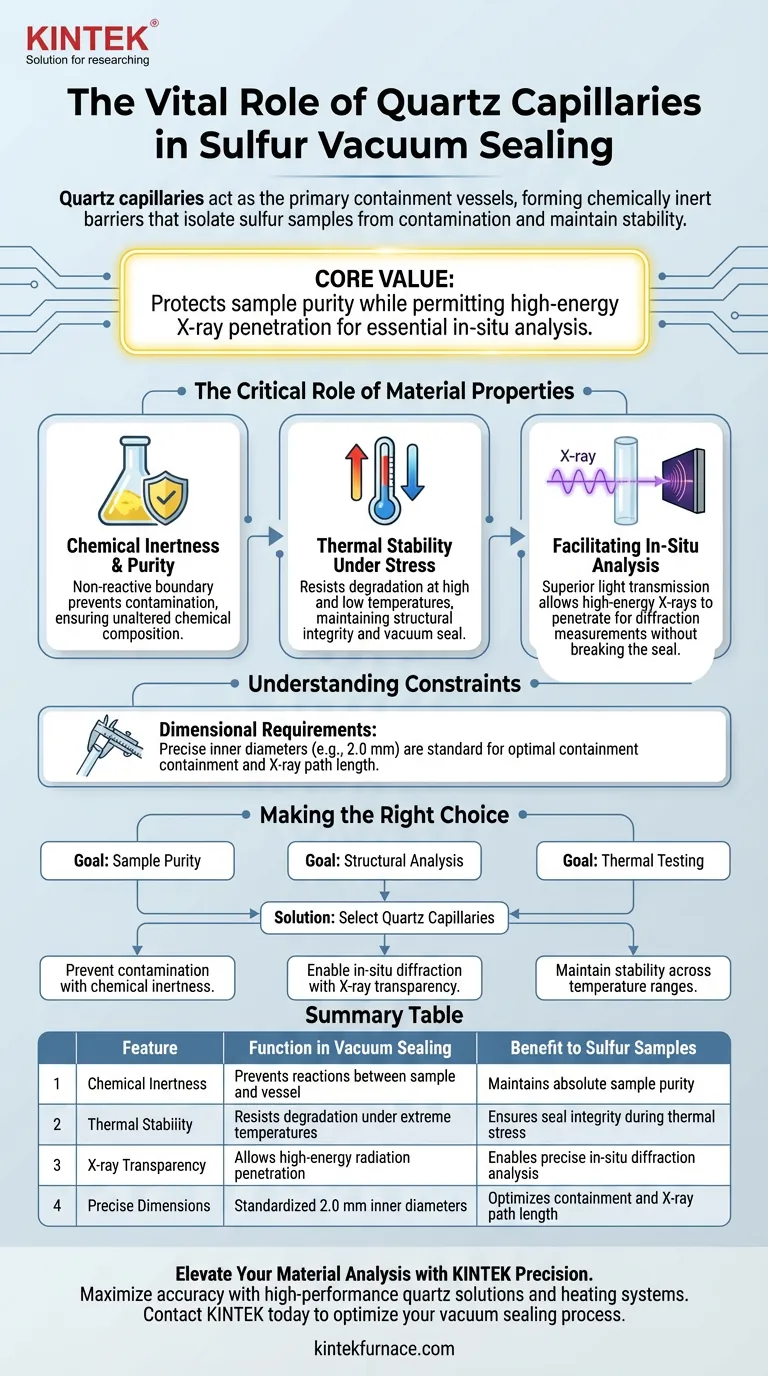
Quartz capillaries serve as the primary containment vessels for sulfur samples during the vacuum sealing process. They act as chemically inert barriers that isolate the sulfur from external contamination while maintaining the sample's physical and chemical stability under varying thermal conditions.
The core value of using quartz lies in its unique ability to protect the sample's purity while simultaneously permitting high-energy X-rays to penetrate the walls for essential in-situ analysis.

The Critical Role of Material Properties
To understand why quartz is the standard for sealing sulfur, one must look at the material's specific physical characteristics. These properties directly address the challenges of handling sulfur in experimental environments.
Chemical Inertness and Purity
The primary function of the quartz capillary is to act as a chemically inert container.
Sulfur samples are often sensitive to impurities. The quartz walls provide a non-reactive boundary that prevents contamination from the container itself.
This ensures that the chemical composition of the sulfur remains unaltered during the sealing process.
Thermal Stability Under Stress
Quartz provides exceptional thermal resistance.
Experimental workflows often subject sulfur samples to both high and low temperatures. The capillary must endure these extremes without degrading or failing.
By maintaining structural integrity, the quartz ensures the vacuum seal holds regardless of thermal fluctuations.
Facilitating In-Situ Analysis
Beyond simple containment, the choice of quartz is driven by the need to analyze the sample without breaking the seal.
Transparency to High-Energy Radiation
Quartz offers superior light transmission characteristics.
This transparency allows high-energy X-rays to penetrate the capillary walls. This is critical for performing in-situ diffraction measurements.
Uncompromised Data Collection
Because the X-rays can pass through the container, researchers can gather diffraction data directly from the sealed sample.
This eliminates the need to expose the sulfur to the environment for measurement, preserving the experimental conditions.
Understanding the Constraints
While quartz is the ideal material for this application, successful implementation relies on strict adherence to physical specifications.
Dimensional Requirements
The effectiveness of the capillary is dependent on specific dimensions.
For example, an inner diameter of 2.0 mm is often cited as a standard specification for these preparations.
Deviating from these dimensions may affect the containment capacity or the path length for X-ray diffraction, potentially compromising the data.
Making the Right Choice for Your Goal
When designing experiments involving sulfur samples, the selection of your capillary defines your analytical capabilities.
- If your primary focus is Sample Purity: Rely on quartz for its chemical inertness to prevent environmental or container-based contamination.
- If your primary focus is Structural Analysis: Utilize quartz for its X-ray transparency, enabling in-situ diffraction without breaking the vacuum seal.
- If your primary focus is Thermal Testing: Depend on the thermal resistance of quartz to maintain containment stability across high and low-temperature ranges.
Select quartz capillaries to ensure your sulfur samples remain stable, pure, and accessible for complex analysis.
Summary Table:
| Feature | Function in Vacuum Sealing | Benefit to Sulfur Samples |
|---|---|---|
| Chemical Inertness | Prevents reactions between sample and vessel | Maintains absolute sample purity |
| Thermal Stability | Resists degradation under extreme temperatures | Ensures seal integrity during thermal stress |
| X-ray Transparency | Allows high-energy radiation penetration | Enables precise in-situ diffraction analysis |
| Precise Dimensions | Standardized 2.0 mm inner diameters | Optimizes containment and X-ray path length |
Elevate Your Material Analysis with KINTEK Precision
Maximize the accuracy of your research with high-performance quartz solutions and heating systems designed for sensitive sulfur analysis. Backed by expert R&D and manufacturing, KINTEK offers high-quality Muffle, Tube, Rotary, Vacuum, and CVD systems, along with customizable lab high-temp furnaces tailored to your unique experimental needs.
Ready to optimize your vacuum sealing process? Contact KINTEK today to discuss how our customizable laboratory equipment can deliver the stability and purity your research demands.
Visual Guide
References
- The Structure of Glassy and Liquid Sulfur Revisited. DOI: 10.52825/glass-europe.v3i.2532
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Ultra High Vacuum Observation Window KF Flange 304 Stainless Steel High Borosilicate Glass Sight Glass
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
People Also Ask
- How does the selection of a ceramic crucible contribute to the preparation of biomass carbon catalysts? Maximize Purity
- What is the temperature range for Laboratory Type Furnaces? Find Your Ideal Heat Solution
- Why is graphite foil used to line graphite molds before loading titanium alloy powder? Ensure Purity and Protect Molds
- What maintenance is required after using the alumina furnace tube? Ensure Longevity and Purity in Your Lab
- What role does a precision drying oven play in the pre-treatment of Bi-Fe oxide powders? Safeguard Your Nano-Morphology
- What are the key advantages of using quartz tubes in high-temperature applications? Achieve Unmatched Thermal Stability and Purity
- Why are precision molds and laboratory presses critical for niobium-doped TiO2 ceramics? Achieve 94% Theoretical Density
- How does a water circulating vacuum pump create negative pressure? Discover the Liquid-Ring Mechanism for Efficient Lab Vacuum








