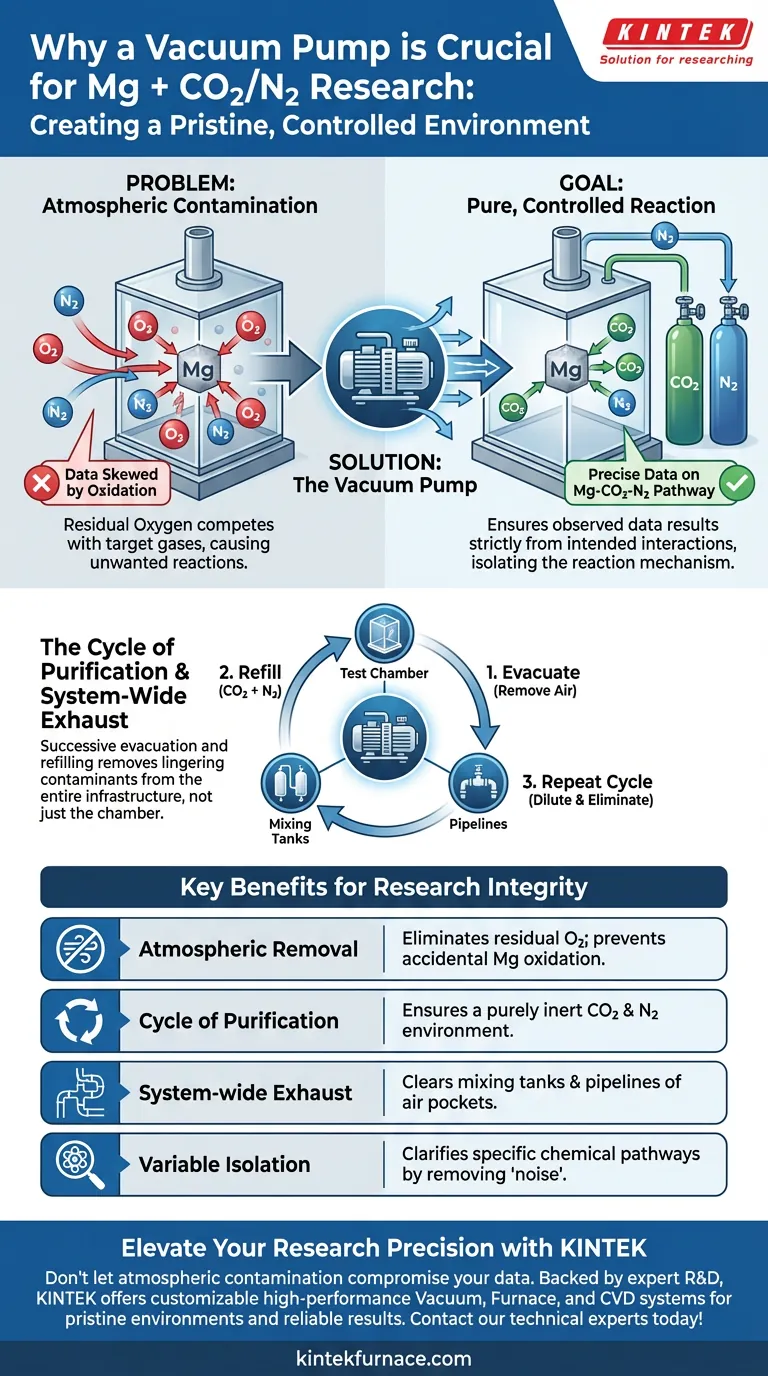
The primary function of a vacuum pump in this specific research context is to establish a pristine, controlled environment by removing atmospheric air before the experiment begins. By thoroughly exhausting the test chamber, mixing tanks, and connecting pipelines, the pump eliminates the presence of oxygen that would otherwise contaminate the study.
Magnesium is highly reactive with oxygen. Using a vacuum pump to systematically remove air ensures that the observed experimental data results strictly from the interaction between magnesium, carbon dioxide, and nitrogen, rather than accidental oxidation with atmospheric oxygen.

Creating a Baseline for Accurate Data
Removing Atmospheric Contaminants
The fundamental challenge in studying magnesium reactions is the metal's high affinity for oxygen found in standard air. If the test environment contains residual air, the magnesium will react with the oxygen rather than the intended gas mixture.
The vacuum pump serves as the first line of defense against this interference. It physically removes the air from the test chamber to prevent competitive reactions that would skew the data.
The Cycle of Purification
Simply running the vacuum once is often not enough to guarantee a purely inert environment. The standard procedure involves a rigorous cycle of evacuating the chamber and then refilling it with the target gases.
By repeating this "evacuate and refill" process, researchers progressively dilute and remove any lingering atmospheric contaminants. This ensures the final environment is composed solely of the intended carbon dioxide and nitrogen mixture.
Isolating the Reaction Mechanism
Focusing on Specific Chemical Pathways
The objective of this research is to uncover the specific mechanisms at play when magnesium reacts with carbon dioxide and nitrogen. To understand these complex interactions, variables must be minimized.
By securing an oxygen-free environment, the vacuum pump allows researchers to isolate the specific chemical pathways of the Mg-CO2-N2 system. This clarity is impossible if the "noise" of oxygen combustion is present.
Clearing the Entire System
Contamination can hide in more places than just the main reaction vessel. The vacuum pump is utilized to exhaust the entire gas delivery infrastructure, including mixing tanks and connecting pipelines.
This holistic approach ensures that no pockets of air are trapped in the tubing, which could be swept into the chamber once the gas flow begins.
Understanding the Trade-offs
Preparation Time vs. Data Integrity
Achieving a truly oxygen-free environment is a time-intensive process. The requirement for multiple cycles of evacuation and refilling extends the setup time for each experiment significantly.
However, this investment of time is non-negotiable for high-fidelity research. Rushing this stage or utilizing a weak vacuum seal introduces a high risk of data invalidation, rendering the subsequent results unreliable.
Ensuring Experimental Integrity
To obtain valid data on magnesium reactions, strict atmospheric control is the single most critical variable.
- If your primary focus is reaction mechanism analysis: Prioritize multiple evacuation cycles to ensure a zero-oxygen baseline, as even trace amounts can alter the chemical pathway.
- If your primary focus is equipment setup: Ensure the vacuum system is connected to all peripheral mixing tanks and pipelines, not just the main chamber, to prevent downstream contamination.
Ultimately, the vacuum pump acts as the gatekeeper of scientific validity, transforming a potentially chaotic open-air reaction into a precise, measurable chemical study.
Summary Table:
| Feature | Purpose in Research | Benefit to Experiment |
|---|---|---|
| Atmospheric Removal | Eliminates residual oxygen | Prevents accidental magnesium oxidation |
| Cycle of Purification | Successive evacuation and refilling | Ensures a pure CO2 and N2 environment |
| System-wide Exhaust | Clears mixing tanks and pipelines | Eliminates air pockets in gas delivery lines |
| Variable Isolation | Removes atmospheric 'noise' | Clarifies specific chemical reaction pathways |
Elevate Your Research Precision with KINTEK
Don't let atmospheric contamination compromise your experimental integrity. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique laboratory requirements. Whether you are studying complex metal reactions or developing new materials, our advanced high-temperature furnaces and vacuum solutions provide the pristine environment you need for reliable, repeatable data.
Ready to optimize your lab setup? Contact our technical experts today to find the perfect solution for your research needs!
Visual Guide
References
- Ioan Barabulica, Ioan Mămăligă. Experimental Study on the Reaction of Magnesium in Carbon Dioxide and Nitrogen Atmosphere. DOI: 10.3390/chemengineering8020041
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
People Also Ask
- Why are alumina or ceramic crucibles selected for KCdCl3 perovskite? Ensure High Purity and Thermal Stability
- What role do quartz tubes and vacuum sealing play in synthesis? Master High-Reactivity Compounds like U0.92Mn3Si2C
- How does a laboratory drying oven contribute to the preparation of C@TiC/SiO2 xerogels? Ensure Structural Integrity
- What are the considerations for using high-purity alumina crucibles or boats for SrVO3 sintering? Best Practices
- Why use high-purity quartz glass tubes for copper sulfide synthesis? Ensure Thermal Stability & Purity
- What happens during the 180-degree rotation of the impeller in a water circulating vacuum pump? Uncover the Suction Mechanism
- What are the functions of a boron nitride (BN) crucible and internal packing powder? Optimize Si3N4 Sintering Now
- Why is the use of high-vacuum pump groups critical for photothermal catalytic chamber pre-treatment?



















