Quartz tubes combined with vacuum sealing technology serve as the fundamental containment system necessary for synthesizing high-reactivity compounds like U0.92Mn3Si2C. This configuration creates a strictly closed, oxygen-free environment that prevents the rapid oxidation of active uranium precursors at elevated temperatures while regulating vapor pressure to ensure the correct chemical ratios are maintained.
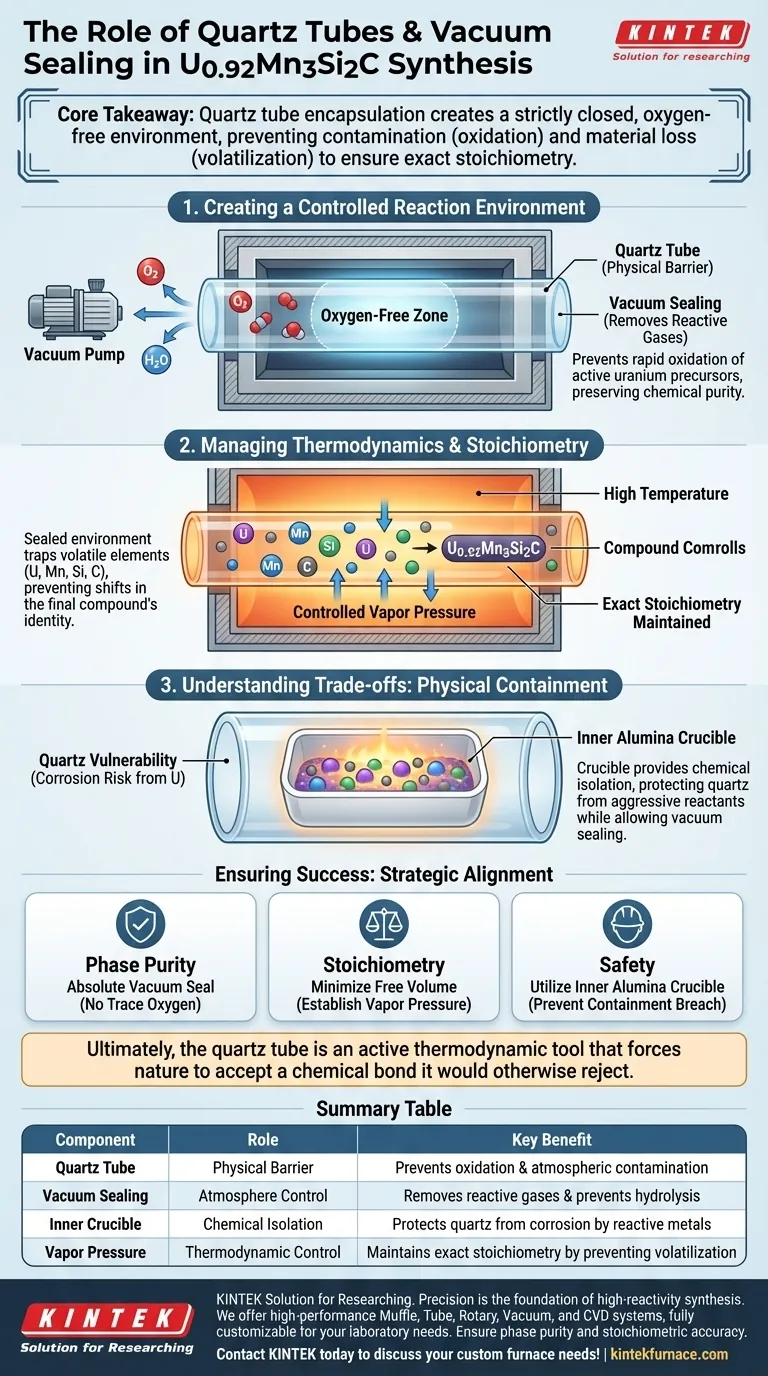
Core Takeaway The synthesis of U0.92Mn3Si2C relies on quartz tube encapsulation to impose an artificial atmospheric boundary. This ensures phase purity by preventing external contamination (oxidation) and internal loss of material (volatilization), guaranteeing the final product matches the intended stoichiometry.

Creating a Controlled Reaction Environment
The Necessity of an Oxygen-Free Zone
For compounds involving highly active elements like uranium, the presence of atmospheric oxygen is detrimental.
Quartz tubes act as a physical barrier, while vacuum sealing removes reactive gases.
This isolation prevents the oxidation of uranium precursors, which would otherwise degrade immediately upon heating.
Preservation of Chemical Purity
High-reactivity synthesis is intolerant of impurities.
By utilizing vacuum sealing technology, you create a system that is strictly closed to the outside world.
This prevents the ingress of moisture or air that could lead to hydrolysis or the formation of unwanted secondary phases.
Managing Thermodynamics and Stoichiometry
Controlling Vapor Pressure
At the high temperatures required for synthesis, certain elements can become volatile and attempt to escape the reaction mixture.
The sealed quartz tube maintains a controlled vapor pressure within the vessel.
This pressure equilibrium forces volatile components to remain part of the reaction rather than evaporating away.
Ensuring Exact Stoichiometry
The precise ratio of elements (U, Mn, Si, C) defines the compound's identity.
If volatile components are lost to evaporation, the stoichiometry shifts, resulting in a failed synthesis.
The sealed environment traps these elements, ensuring the final product, U0.92Mn3Si2C, maintains the exact chemical composition intended.
Understanding the Trade-offs: Physical Containment
The Vulnerability of Quartz
While quartz provides an excellent atmospheric seal, it is not chemically invincible.
Direct contact between the quartz wall and aggressive precursors, such as metallic uranium or molten fluxes, can lead to severe corrosion or vessel failure.
At high temperatures, these reactive metals can attack the silica in the quartz, compromising the vacuum seal.
The Role of Inner Crucibles
To mitigate quartz corrosion, the system often requires a secondary layer of protection.
High-purity alumina crucibles are frequently placed inside the quartz tube to physically hold the raw materials.
This setup provides necessary chemical inertness, isolating the aggressive reactants from the quartz walls while still allowing the quartz tube to perform its primary function of vacuum sealing.
Ensuring Success in High-Temperature Synthesis
To achieve high-quality results with compounds like U0.92Mn3Si2C, align your containment strategy with your specific chemical risks:
- If your primary focus is Phase Purity: Ensure the vacuum seal is absolute to prevent even trace amounts of oxygen from oxidizing the uranium precursors.
- If your primary focus is Stoichiometry: Minimize the free volume inside the quartz tube to establish equilibrium vapor pressure quickly and prevent material loss.
- If your primary focus is Safety: Utilize an inner alumina crucible to prevent reactive metals from breaching the quartz containment vessel.
Ultimately, the quartz tube is not just a container; it is an active thermodynamic tool that forces nature to accept a chemical bond it would otherwise reject.
Summary Table:
| Component | Role in Synthesis | Key Benefit |
|---|---|---|
| Quartz Tube | Physical Barrier | Prevents oxidation & atmospheric contamination |
| Vacuum Sealing | Atmosphere Control | Removes reactive gases & prevents hydrolysis |
| Inner Crucible | Chemical Isolation | Protects quartz from corrosion by reactive metals |
| Vapor Pressure | Thermodynamic Control | Maintains exact stoichiometry by preventing volatilization |
Precision is the foundation of high-reactivity synthesis. Whether you are developing advanced alloys or complex compounds like U0.92Mn3Si2C, KINTEK provides the specialized equipment needed for success. Backed by expert R&D and manufacturing, we offer high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique laboratory requirements. Ensure phase purity and stoichiometric accuracy in every experiment. Contact KINTEK today to discuss your custom furnace needs!
Visual Guide
References
- Hope A. Long, Vladislav V. Klepov. Synthesis of U<sub>0.92</sub>Mn<sub>3</sub>Si<sub>2</sub>C Using Organic Carbon Source. DOI: 10.1002/zaac.202500047
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What is the technical objective of using vacuum-sealed quartz capsules for Co-Ti-V alloy homogenization? Safeguard Chemical Integrity
- How does the design of a graphite box optimize the sulfurization of Sb thin films? Key Insights for Film Uniformity
- What are the roles of laboratory vacuum drying ovens and precision analytical balances in moisture monitoring?
- How do vacuum systems assist in pure thin film growth? Enhance PLD Chemical Purity with Turbo-Molecular Pumps
- Why are high-purity alumina crucibles used for MAX phase sintering? Ensure Purity in High-Temperature Synthesis
- What is the importance of using spot-welded K-type thermocouples in DP steel heat treatment? Master Thermal Precision
- Why use high-purity quartz glass tubes for copper sulfide synthesis? Ensure Thermal Stability & Purity
- How do vacuum filtration systems operate in industrial sludge dehydration? Achieve Efficient Liquid-Solid Separation



















