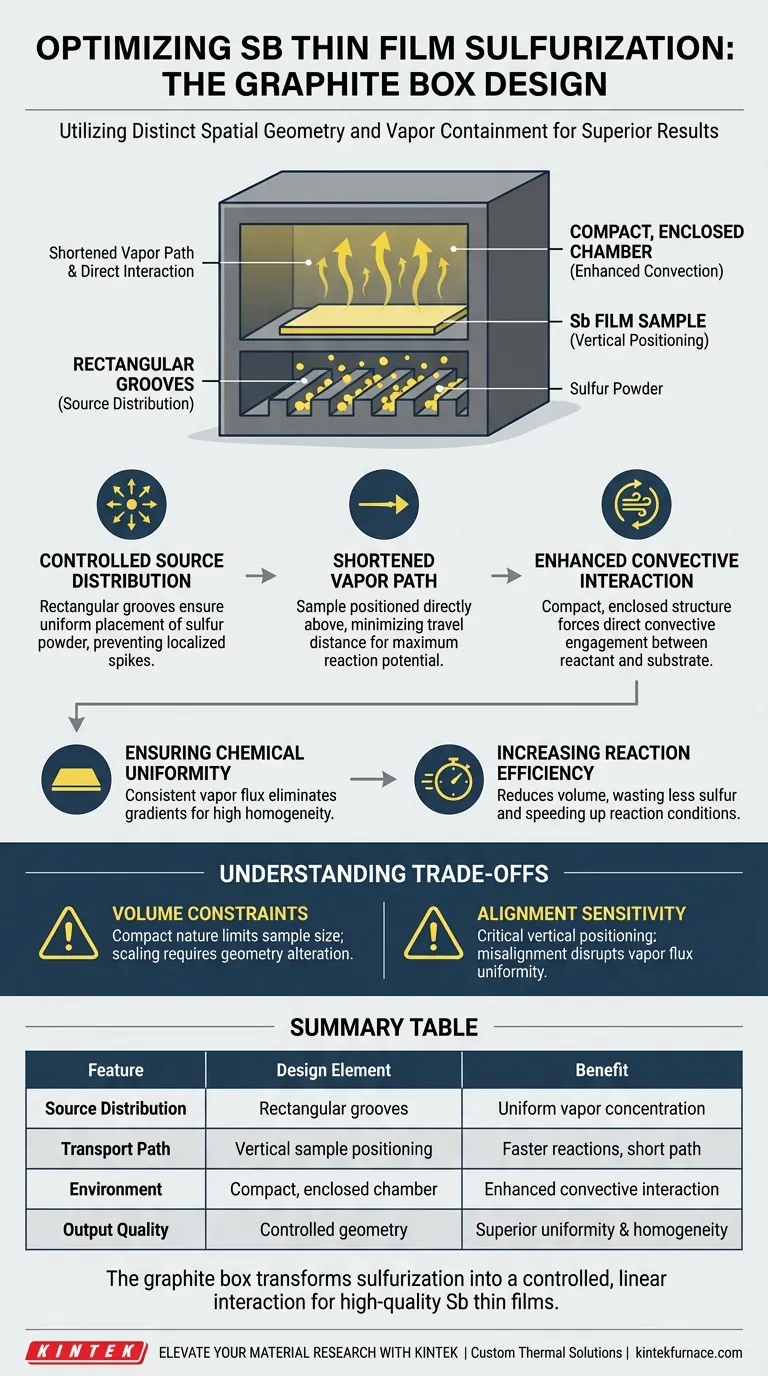
The design of a graphite box optimizes the sulfurization of antimony (Sb) thin films primarily through distinct spatial geometry and vapor containment. By featuring rectangular grooves at the base for sulfur powder and positioning the film sample directly above, the box creates a compact environment. This setup shortens the distance sulfur vapor must travel, facilitating a direct and efficient reaction.
The graphite box utilizes a compact, enclosed geometry with basal grooves to create a controlled convective environment. This design minimizes the vapor transport path, ensuring consistent chemical distribution and high uniformity across the antimony thin film.

The Mechanics of the Graphite Box Design
To understand why this specific design yields better results, we must look at how the physical structure influences the chemical transport of sulfur.
Controlled Source Distribution
The foundation of the box features rectangular grooves.
These grooves are not merely for storage; they ensure the uniform placement of the sulfur powder. By spreading the source material evenly across the base, the design prevents localized spikes in sulfur concentration, which could lead to uneven reaction rates.
Shortened Vapor Transport Path
The antimony film sample is positioned directly above the sulfur powder.
In larger or open systems, vapor must travel significant distances, often leading to dissipation or cooling. This compact design drastically shortens the contact path, ensuring that the vapor reaches the metallic film with maximum potential for reaction.
Enhanced Convective Interaction
The structure is explicitly enclosed and compact.
This containment forces the rising sulfur vapor to interact with the film through convective interaction. Rather than relying on passive diffusion in a large chamber, the box forces a direct engagement between the reactant and the substrate.
The Impact on Film Quality
The physical design translates directly into the chemical quality of the final product.
Ensuring Chemical Uniformity
The primary output of this design is chemical uniformity.
Because the sulfur source is evenly distributed and the transport path is short and direct, the entire surface of the antimony film receives a consistent flux of sulfur vapor. This eliminates gradients where some parts of the film might be over-sulfurized while others remain metallic.
Increasing Reaction Efficiency
The direct vertical alignment facilitates a more efficient reaction.
By reducing the volume the vapor must fill before contacting the sample, the system wastes less sulfur and achieves the necessary reaction conditions more rapidly than non-optimized setups.
Understanding the Trade-offs
While the graphite box design offers significant advantages for uniformity and efficiency, it introduces specific constraints inherent to its geometry.
Volume Constraints
The effectiveness of this design relies on its compact nature.
This implies a physical limit on the size of the samples that can be processed. Scaling this specific "short-path" design to significantly larger substrates may require altering the geometry, which could negatively impact the convective efficiency described.
Alignment Sensitivity
The system relies on the sample being positioned directly above the grooves.
This vertical alignment is critical. Any misalignment or tilting of the sample relative to the grooves could disrupt the uniformity of the vapor flux, negating the benefits of the groove design.
Making the Right Choice for Your Goal
The graphite box is a specialized tool designed to solve specific problems regarding uniformity and transport.
- If your primary focus is film homogeneity: Rely on the rectangular groove design to ensure the sulfur source is evenly distributed beneath the entire sample surface.
- If your primary focus is reaction speed: Utilize the compact, enclosed structure to minimize vapor travel time and maximize convective transfer.
Ultimately, the graphite box transforms sulfurization from a chaotic vapor process into a controlled, linear interaction that guarantees high-quality antimony thin films.
Summary Table:
| Feature | Design Element | Benefit to Sulfurization |
|---|---|---|
| Source Distribution | Rectangular grooves at base | Ensures uniform sulfur vapor concentration |
| Transport Path | Vertical sample positioning | Shortens vapor travel distance for faster reactions |
| Environment | Compact, enclosed chamber | Enhances convective interaction and prevents dissipation |
| Output Quality | Controlled geometry | Achieves superior chemical uniformity and film homogeneity |
Elevate Your Material Research with KINTEK
Precise sulfurization requires more than just high temperatures; it demands a controlled environment. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to accommodate specialized graphite box geometries for your unique thin-film needs. Whether you are scaling Sb-based semiconductors or optimizing lab-scale reactions, our engineering team ensures you have the right high-temp furnace to achieve perfect chemical uniformity.
Ready to optimize your thermal processes? Contact us today to discuss your custom solution!
Visual Guide
References
- Sheyda Uc-Canché, Juan Luis Ruiz de la Peña. Influence of Sulfurization Time on Sb2S3 Synthesis Using a New Graphite Box Design. DOI: 10.3390/ma17071656
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What is the necessity of using a laboratory vacuum drying oven when processing Fe-N-C catalyst powders?
- How does a lab vacuum pump work? Understanding the Liquid Piston Mechanism
- Why is a heat-resistant crucible indispensable for magnesium purification? Ensuring Purity and Efficiency in Vacuum Sublimation
- Why is a Mass Flow Controller (MFC) important for gas-phase corrosion research? Ensure Data Integrity & Precision
- Why are high-purity alumina crucibles used for containing molten high-silicon steel? Ensure Purity & Thermal Stability
- Why is a Boron Nitride coating applied to graphite crucibles for Mg3Sb2 alloys? Protect Purity and Tooling
- Why are high-alumina crucibles required for static immersion corrosion tests? Ensure Data Purity at 1000°C
- What is the function of high-purity quartz encapsulation tubes? Key Roles in Chalcogenide Glass Synthesis



















