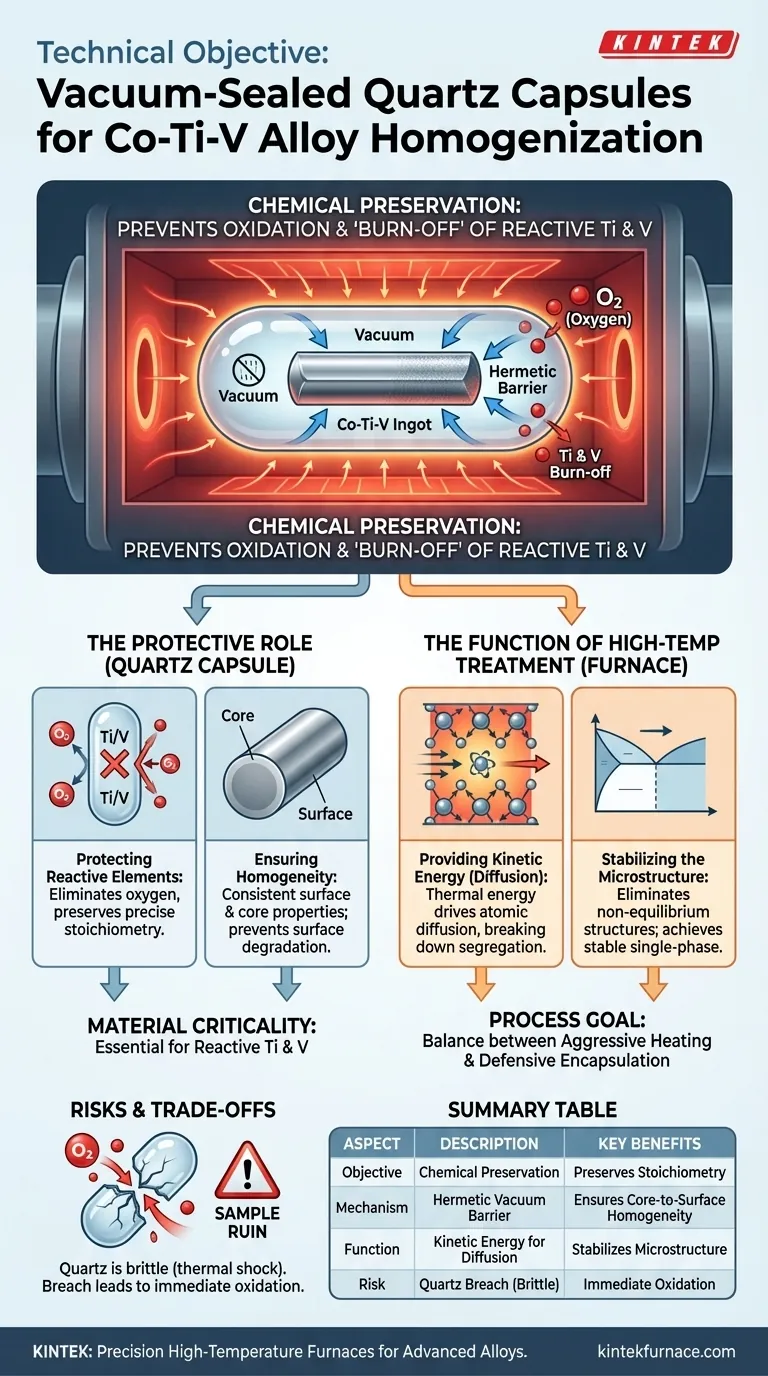
The technical objective is fundamentally about chemical preservation during thermal stress. By enclosing Co-Ti-V alloy ingots in vacuum-sealed quartz capsules, you establish a hermetic barrier that withstands temperatures as high as 1100 °C. This isolation prevents the oxidation and subsequent "burn-off" of highly reactive elements—specifically Titanium (Ti) and Vanadium (V)—during prolonged heat treatment cycles.
The quartz capsule acts as a sacrificial shield, maintaining a vacuum or inert environment around the ingot. This ensures that the chemical composition of the alloy's surface remains identical to its core, preventing surface degradation that would otherwise occur due to air exposure at elevated temperatures.
The Critical Role of Elemental Isolation
Protecting Reactive Elements
Titanium and Vanadium are classified as active elements. When exposed to oxygen at high temperatures, they oxidize rapidly.
In an open furnace environment, these elements would "burn off" or degrade. The vacuum-sealed quartz capsule creates a micro-environment that eliminates oxygen presence, preserving the precise stoichiometry of your alloy.
Ensuring Homogeneity from Core to Surface
The goal of homogenization is consistency. If the surface oxidizes, the material properties at the exterior will differ significantly from the interior.
The quartz barrier ensures that the performance characteristics of the alloy surface remain consistent with the bulk material. This allows for accurate testing and application of the alloy post-treatment.
The Function of High-Temperature Treatment
Providing Kinetic Energy for Diffusion
While the capsule protects, the furnace heat drives the process. The high-temperature environment (e.g., 1100 °C) provides the necessary thermal energy for atoms to move.
This kinetic energy enables atoms within the alloy to diffuse effectively. This diffusion is essential for breaking down the segregation that occurs during the initial melting phase.
Stabilizing the Microstructure
The ultimate goal of this thermal cycle is to eliminate non-equilibrium structures.
By maintaining high heat for extended periods (up to 48 hours) inside the capsule, the alloy achieves a stable, single-phase solid solution structure. This is particularly critical for medium and high entropy alloys where structural stability is paramount.
Understanding the Trade-offs
The Limitations of Quartz
While quartz offers excellent high-temperature resistance and sealing properties, it is not indestructible. It is brittle and subject to thermal shock if cooled or heated too rapidly.
The Risk of Breach
The integrity of the process relies entirely on the seal. If the quartz capsule fails or leaks during the 48-hour cycle, oxygen will infiltrate the environment immediately.
This would result in the exact oxidation and elemental loss the process was designed to prevent, likely ruining the sample.
Making the Right Choice for Your Goal
To maximize the effectiveness of your homogenization process, consider the following specific objectives:
- If your primary focus is compositional accuracy: Prioritize a high-quality vacuum seal to prevent even trace amounts of oxygen from reacting with the Titanium and Vanadium.
- If your primary focus is structural uniformity: Ensure the furnace temperature is maintained consistently (e.g., 1100 °C) for the full duration to allow sufficient atomic diffusion throughout the ingot.
Successful homogenization relies on the balance between aggressive heating to drive diffusion and defensive encapsulation to preserve chemistry.
Summary Table:
| Aspect | Description |
|---|---|
| Primary Objective | Chemical preservation; prevent oxidation and 'burn-off' of reactive elements (Ti, V) during high-temperature thermal stress. |
| Mechanism | Creates a hermetic, vacuum-sealed barrier around the ingot, isolating it from oxygen at temperatures up to 1100 °C. |
| Key Benefits | Preserves precise stoichiometry, ensures core-to-surface homogeneity, enables efficient atomic diffusion, stabilizes microstructure. |
| Material Criticality | Essential for reactive elements like Titanium (Ti) and Vanadium (V) in Co-Ti-V alloys. |
| Limitations/Risks | Quartz is brittle (thermal shock risk); capsule breach during treatment leads to immediate oxidation and sample ruin. |
Achieve unparalleled material integrity for your advanced alloys with KINTEK's precision high-temperature furnaces. Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, CVD systems, and other lab high-temp furnaces, all customizable for unique needs. Ensure your homogenization processes, like those for Co-Ti-V alloys, consistently deliver precise chemical and structural uniformity. Contact us today to optimize your thermal processing solutions.
Visual Guide
References
- The Effect of Nb on the Microstructure and High-Temperature Properties of Co-Ti-V Superalloys. DOI: 10.3390/coatings15010053
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
People Also Ask
- What is the importance of using spot-welded K-type thermocouples in DP steel heat treatment? Master Thermal Precision
- How do a brass cap and a cooling element work together? Ensuring Reliable High-Temperature Experimental Seals
- What role does a PTFE-lined high-pressure autoclave play in synthesis of ZnO nanorods? Key Benefits & Growth Factors
- What is the function of high-purity alumina crucibles? Protect Samples and Furnaces During Oxide Calcination
- Are customization options available for alumina ceramic furnace tubes? Tailor Them for Your Lab's Needs
- What necessary conditions does a vacuum chamber provide for vapor deposition? Achieve High-Purity Nanofluid Synthesis
- What is the purpose of using quartz vacuum encapsulation? Optimize La(Fe,Si)13-based Magnetocaloric Alloys
- What customization options are available for alumina ceramic tubes? Tailor for High-Temp, Corrosion-Resistant Applications



















