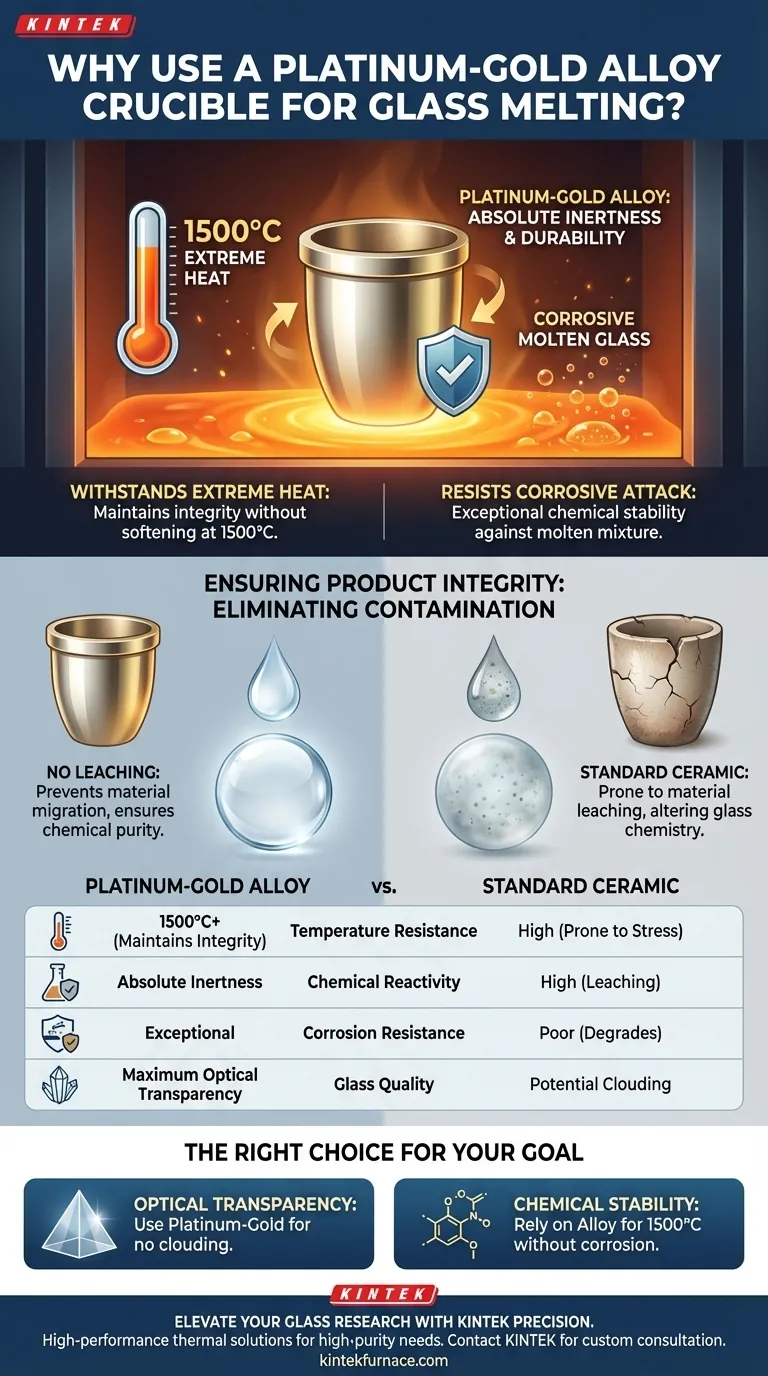
A platinum-gold alloy crucible is utilized primarily for its ability to withstand extreme thermal conditions while maintaining absolute chemical inertness. Operating at temperatures as high as 1500°C, this specific alloy resists the corrosive nature of molten glass to prevent contamination. It is the material of choice when the optical and chemical integrity of the final product is non-negotiable.
The core advantage of using a platinum-gold alloy is its refusal to react with the melt. By eliminating the material leaching common in standard vessels, it guarantees the chemical purity and optical transparency required for high-quality silicate glass.

Overcoming Thermal and Chemical Challenges
Withstanding Extreme Heat
The glass melting process requires an operating environment capable of reaching 1500°C.
A platinum-gold alloy possesses an extremely high melting point. This allows the vessel to maintain its structural integrity without softening or deforming under intense thermal stress.
Resisting Corrosive Attack
Molten glass is not just hot; it is a strong corrosive agent.
Standard materials often degrade when exposed to this aggressive chemical environment. The platinum-gold alloy offers exceptional chemical stability, effectively resisting attack from the molten mixture.
Ensuring Product Integrity
Eliminating Contamination
The most significant risk in glass production is the introduction of impurities from the crucible itself.
Unlike other materials, the platinum-gold alloy prevents material leaching. This ensures that no trace metals or foreign substances migrate from the vessel wall into the glass melt.
Preserving Optical Quality
For silicate glass, clarity is often the ultimate measure of success.
By maintaining a pure melt, the alloy ensures the final product achieves high optical transparency. This makes it indispensable for applications where visual defects or clouding are unacceptable.
Understanding the Trade-offs
Alloy vs. Ceramic Crucibles
The primary alternative to platinum-gold is the standard ceramic crucible.
While ceramics are widely used, they lack the necessary resistance for high-purity applications. They are prone to leaching materials into the melt, which fundamentally alters the chemistry of the glass.
The Cost of Purity
The choice of crucible dictates the quality ceiling of the production run.
If you choose standard ceramics, you accept a baseline level of contamination. The platinum-gold alloy is a specialized tool used specifically to avoid this compromise.
Making the Right Choice for Your Goal
Selecting the correct crucible depends entirely on the tolerance for impurities in your final product.
- If your primary focus is optical transparency: Use platinum-gold to ensure no foreign materials leach into the silicate glass and cloud the result.
- If your primary focus is chemical stability: Rely on this alloy to withstand temperatures of 1500°C without corroding into the melt.
The platinum-gold crucible is the definitive solution for processing molten glass when purity is the priority.
Summary Table:
| Feature | Platinum-Gold Alloy Crucible | Standard Ceramic Crucible |
|---|---|---|
| Temperature Resistance | Up to 1500°C+ (Maintains Integrity) | High (But prone to thermal stress) |
| Chemical Reactivity | Absolute Inertness | High (Prone to material leaching) |
| Corrosion Resistance | Exceptional against molten glass | Poor (Degrades over time) |
| Glass Quality | Maximum Optical Transparency | Potential Clouding/Impurities |
| Best Use Case | High-purity Silicate Glass | General Industrial Glass |
Elevate Your Glass Research with KINTEK Precision
Don't let crucible contamination compromise your material integrity. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, and Vacuum systems alongside specialized lab solutions tailored for your unique high-temperature needs. Whether you are melting high-purity silicate glass or developing advanced ceramics, our customizable equipment ensures the precision your work demands.
Ready to optimize your thermal processes? Contact KINTEK today for a custom consultation.
Visual Guide
References
- I. M. Teixeira, J. W. Menezes. Transforming Rice Husk Ash into Road Safety: A Sustainable Approach to Glass Microsphere Production. DOI: 10.3390/ceramics8030093
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- Why are flexible graphite gaskets utilized for sealing in LiF-BeF2 molten salt experiments? High-Resilience Solutions
- Why is a high-performance vacuum pumping system required for DLC coatings? Achieve 3.0 x 10^-5 Pa Purity
- How does Energy Dispersive X-ray Spectroscopy (EDX) assist in adjusting furnace parameters? Biochar Quality Control
- Why is an alumina crucible used for vacuum carbothermal reduction? Ensure Purity at 1723 K
- How does a Rapid Thermal Annealing (RTA) system differ from a standard hotplate? Optimize Perovskite Crystallization
- Why is a mass flow controller essential in the tracer method? Precision Data for Pyrolysis Gas Flow
- What is the function of a laboratory pellet press in PCM preparation? Optimize Building Energy Storage Materials
- What is the role of a quartz reactor within a vacuum distillation apparatus for metal recovery? Unlocking Efficient High-Purity Extraction



















