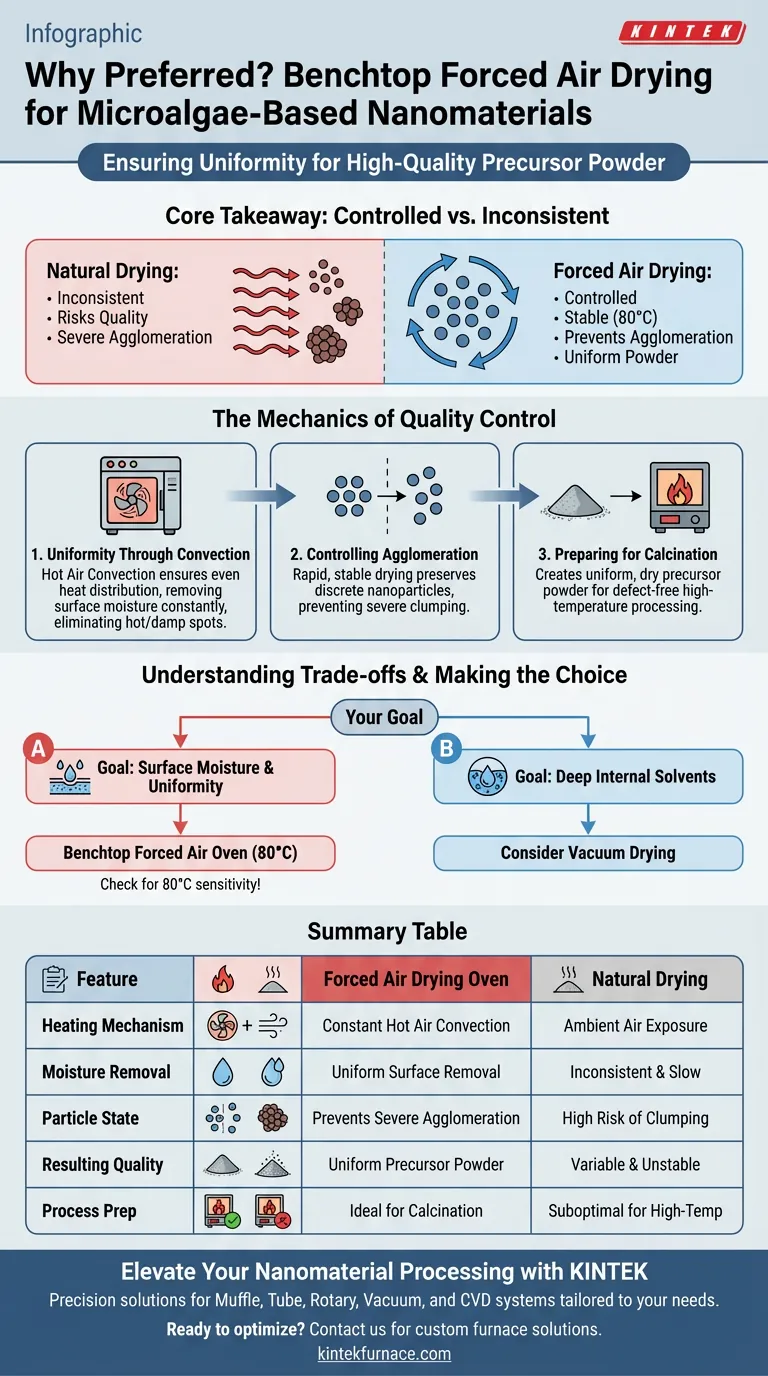
The benchtop forced air drying oven is the preferred choice for drying microalgae-based nanomaterials because it utilizes constant temperature hot air convection to ensure uniform surface moisture removal. By maintaining a stable environment, typically around 80 °C, it prevents the inconsistencies associated with natural drying and produces a high-quality precursor powder ready for subsequent processing.
Core Takeaway While natural drying is inconsistent and risks product quality, forced air drying provides a controlled, stable thermal environment. Its primary advantage is the prevention of severe particle agglomeration, ensuring the creation of a uniform precursor powder essential for successful calcination.

The Mechanics of Quality Control
Achieving Uniformity Through Convection
The defining feature of this equipment is the use of hot air convection. unlike static heating methods, forced air ensures that heat is distributed evenly throughout the chamber.
This mechanism removes moisture from the surface of the nanoparticles at a constant rate. The consistency of this airflow eliminates "hot spots" or damp pockets that often occur with uneven drying methods.
Controlling Particle Agglomeration
One of the most critical challenges in processing nanomaterials is the tendency for particles to clump together, or agglomerate.
The forced air drying oven specifically addresses this by providing a rapid, stable drying environment. By moving moisture away from the particle surface efficiently, it prevents severe particle agglomeration, preserving the discrete nature of the nanomaterials better than natural drying methods.
Preparing for Calcination
The drying phase is rarely the final step; it is a preparatory stage for calcination (high-temperature processing).
To achieve a high-quality final product, the input material—the precursor powder—must be uniform and dry. The forced air oven ensures the powder achieves the necessary physical state to undergo calcination without structural defects.
Understanding the Trade-offs
Surface vs. Deep Solvent Removal
It is important to recognize that forced air drying is optimized for removing surface moisture and preventing agglomeration through convection.
However, if your material contains deep internal solvent residues or is highly sensitive to oxidation, this method has limits. Other methods, such as vacuum drying, are specifically designed to lower boiling points for internal solvent removal and protect against oxidation, whereas forced air relies on thermal convection at higher standard pressures.
Temperature Sensitivity
The typical operating temperature for this phase is 80 °C.
While effective for most microalgae-based precursors, you must ensure your specific biological material does not degrade at this specific temperature threshold before the calcination stage.
Making the Right Choice for Your Goal
Select your drying method based on the specific physical requirements of your precursor material.
- If your primary focus is producing a uniform precursor powder: Use the benchtop forced air oven to prevent agglomeration and prepare for calcination.
- If your primary focus is removing deep internal solvents: Consider investigating vacuum drying options to lower boiling points and target internal residues.
By controlling the drying environment with forced air convection, you transform a variable biological slurry into a stable, high-quality engineering material.
Summary Table:
| Feature | Forced Air Drying Oven | Natural Drying |
|---|---|---|
| Heating Mechanism | Constant Hot Air Convection | Ambient Air Exposure |
| Moisture Removal | Uniform Surface Removal | Inconsistent & Slow |
| Particle State | Prevents Severe Agglomeration | High Risk of Clumping |
| Resulting Quality | Uniform Precursor Powder | Variable & Unstable |
| Process Prep | Ideal for Calcination | Suboptimal for High-Temp |
Elevate Your Nanomaterial Processing with KINTEK
Precision is the foundation of high-quality nanomaterial synthesis. Backed by expert R&D and world-class manufacturing, KINTEK provides high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, along with specialized lab furnaces tailored to your microalgae processing needs. Whether you require precise forced air convection for uniform precursors or vacuum drying for deep solvent removal, our customizable solutions ensure your research and production meet the highest standards.
Ready to optimize your drying and calcination workflow? Contact us today to speak with our technical experts about a custom furnace solution.
Visual Guide
References
- Agnieszka Sidorowicz, Günther Rupprechter. Microalgae-derived Co<sub>3</sub>O<sub>4</sub> nanomaterials for catalytic CO oxidation. DOI: 10.1039/d4ra00343h
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- How does the temperature difference contribute to the cracking of the alumina furnace tube? Prevent Cracks for Longer Tube Life
- What manufacturing processes rely on laboratory furnaces? Precision Heat Treatment for Advanced Materials
- Why are laboratory furnaces considered essential in industrial and scientific applications? Unlock Precision and Control for Your Materials
- Why are desiccators containing saturated salt solutions used when evaluating the hygroscopicity of modified wood?
- How does a vacuum pump facilitate the synthesis process of rare earth-based halide electrolytes? Boost Chemical Purity
- How does the purity of alumina ceramic tubes compare to quartz ceramic tubes? Discover Key Differences for Your Lab
- Why are quartz tubes suitable for material research applications? Ensure Purity and Precision in High-Temp Experiments
- Is it possible to tailor high-temperature laboratory furnaces? Custom Engineering for Unique Research Needs



















