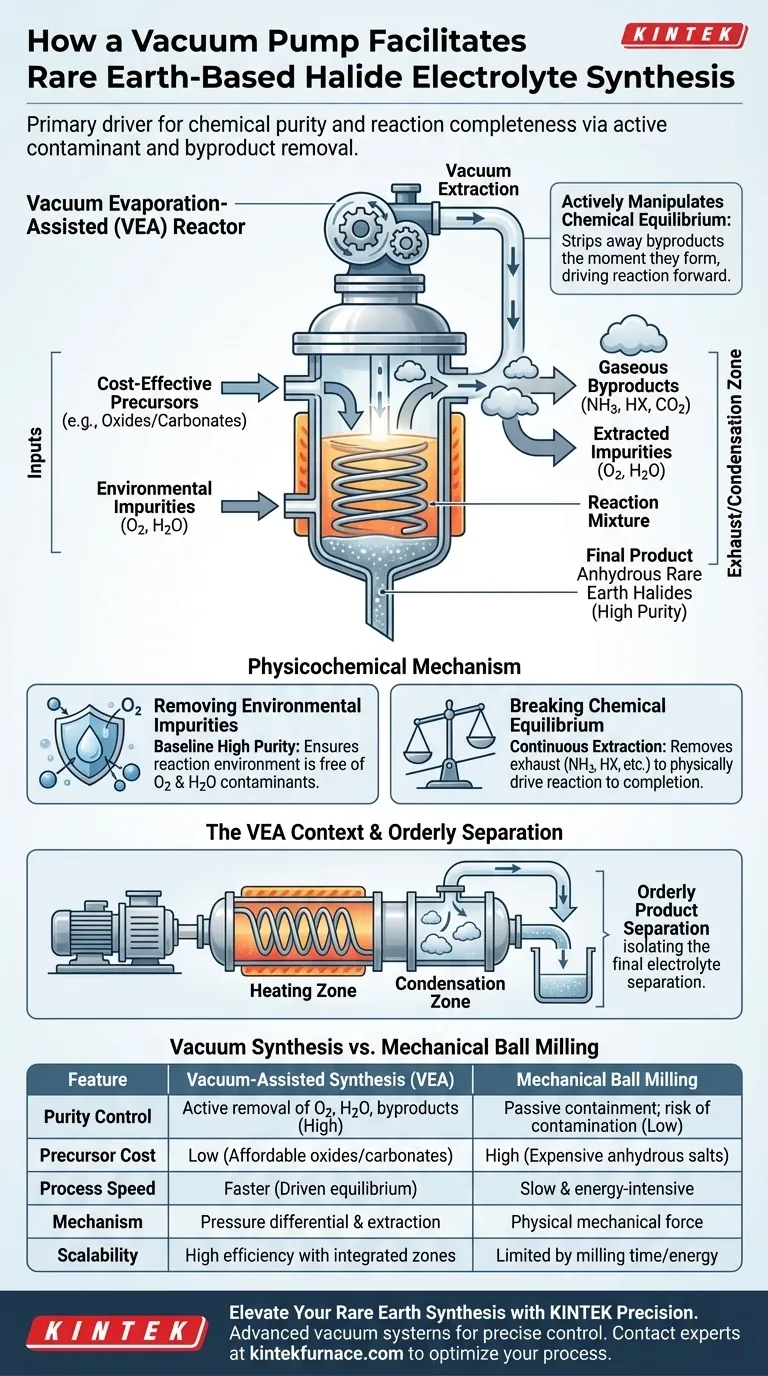
The vacuum pump acts as the primary driver for both chemical purity and reaction completeness. It establishes a critical low-pressure environment that actively removes environmental contaminants like oxygen and water vapor. Furthermore, by continuously extracting gaseous byproducts such as NH3, HX, and CO2 during high-temperature phases, the pump forces the reaction to proceed until anhydrous rare earth halides are fully formed.
The vacuum pump facilitates synthesis not merely by creating a sealed environment, but by actively manipulating chemical equilibrium. By stripping away byproducts the moment they form, it drives the reaction forward, enabling the use of cost-effective precursors while ensuring a high-purity, anhydrous output.

The Physicochemical Mechanism
Removing Environmental Impurities
For rare earth-based halide electrolytes, moisture and oxygen are critical contaminants that degrade performance. A vacuum pump ensures the reaction environment is free of these elements before the process begins. This establishes a baseline of high purity that passive containment cannot achieve.
Breaking Chemical Equilibrium
During the high-temperature reaction phase, the process generates gaseous byproducts, including NH3, HX, H2O, and CO2. If these gases remain in the reaction vessel, the chemical equilibrium stabilizes, preventing the reaction from finishing.
The vacuum pump continuously extracts these gases. By removing the "exhaust," the system breaks this equilibrium, physically driving the chemical reaction toward the complete formation of anhydrous rare earth halides.
The Vacuum Evaporation-Assisted (VEA) Context
Integration with Reactor Zones
The vacuum pump is rarely used in isolation; it is the core component of the Vacuum Evaporation-Assisted (VEA) reactor. This system integrates the vacuum with specific heating and condensation zones. This architecture allows for precise control over the reaction environment and the state of the materials.
Orderly Product Separation
Within a VEA reactor, the vacuum facilitates the orderly separation of synthesized products from byproducts. This ensures that the final electrolyte material is isolated efficiently, streamlining what was traditionally a chaotic separation process.
Operational Trade-offs and Comparisons
Vacuum Synthesis vs. Mechanical Ball Milling
Traditional synthesis often relies on mechanical ball milling. However, this method is energy-intensive and notoriously time-consuming. Ball milling physically forces materials together rather than using pressure differentials to drive chemical changes.
Cost Implications of Precursors
A major limitation of mechanical milling is the requirement for expensive anhydrous salt precursors. Because the vacuum process actively removes water vapor generated during the reaction, it allows for the use of cheaper raw materials, such as rare earth oxides or carbonates.
Complexity vs. Efficiency
While a vacuum system introduces equipment complexity (pumps, seals, condensation zones), it drastically reduces overall raw material costs and processing time. The trade-off is an initial investment in improved reactor infrastructure in exchange for long-term operational efficiency.
Making the Right Choice for Your Goal
To maximize the benefits of a vacuum-assisted synthesis process, align your configuration with your specific production targets:
- If your primary focus is Chemical Purity: Prioritize a high-performance vacuum system to ensure the absolute removal of oxygen and water vapor, guaranteeing an anhydrous final product.
- If your primary focus is Cost Reduction: Leverage the vacuum's ability to handle water vapor by sourcing cheaper rare earth oxides or carbonates instead of pre-processed anhydrous salts.
By shifting from mechanical force to vacuum-driven equilibrium control, you achieve a synthesis process that is faster, cheaper, and chemically superior.
Summary Table:
| Feature | Vacuum-Assisted Synthesis (VEA) | Mechanical Ball Milling |
|---|---|---|
| Purity Control | Active removal of O2, H2O, and gaseous byproducts | Passive containment; risk of contamination |
| Precursor Cost | Low (uses affordable oxides/carbonates) | High (requires expensive anhydrous salts) |
| Process Speed | Faster due to driven chemical equilibrium | Slow and energy-intensive |
| Mechanism | Pressure differential & byproduct extraction | Physical mechanical force |
| Scalability | High efficiency with integrated reactor zones | Limited by milling time and energy consumption |
Elevate Your Rare Earth Synthesis with KINTEK Precision
Don't let chemical equilibrium limit your material performance. KINTEK’s advanced vacuum systems and high-temperature furnaces are engineered to provide the precise pressure control and thermal stability required for superior halide electrolyte production.
Backed by expert R&D and manufacturing, we offer customizable Muffle, Tube, Rotary, Vacuum, and CVD systems designed to reduce your raw material costs while maximizing output purity.
Ready to optimize your lab's synthesis process? Contact our technical experts today to find the perfect thermal processing solution for your unique research needs.
Visual Guide
References
- Zhichao Zeng, Yaping Du. Vacuum evaporation-assisted reaction: sustainable solution for application of rare earth-based halide solid-state electrolytes. DOI: 10.1039/d5sc00003c
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Ultra Vacuum Electrode Feedthrough Connector Flange Power Lead for High Precision Applications
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Hot Press Furnace Machine for Lamination and Heating
People Also Ask
- Is it possible to tailor high-temperature laboratory furnaces? Custom Engineering for Unique Research Needs
- What is the maximum vacuum capacity of the water circulating vacuum pump? Uncover Its Ideal Lab Applications
- Why use a high-purity alumina crucible with a lid for LATP sintering? Ensure Optimal Stoichiometric Stability
- What are alumina ceramic tubes and why are they considered advanced ceramics? Discover High-Performance Solutions for Extreme Environments
- What should be evaluated when assessing supplier reliability for alumina ceramic furnace tubes? Ensure Consistent Performance and Support
- Why are high-purity alumina crucibles used for containing molten high-silicon steel? Ensure Purity & Thermal Stability
- Why are evaporators and condensers required for zirconium tetrachloride purification? Mastering Nuclear-Grade Standards
- Why is a gas mixing system essential for syngas annealing in copper powder production? Ensure Precise Embrittlement



















