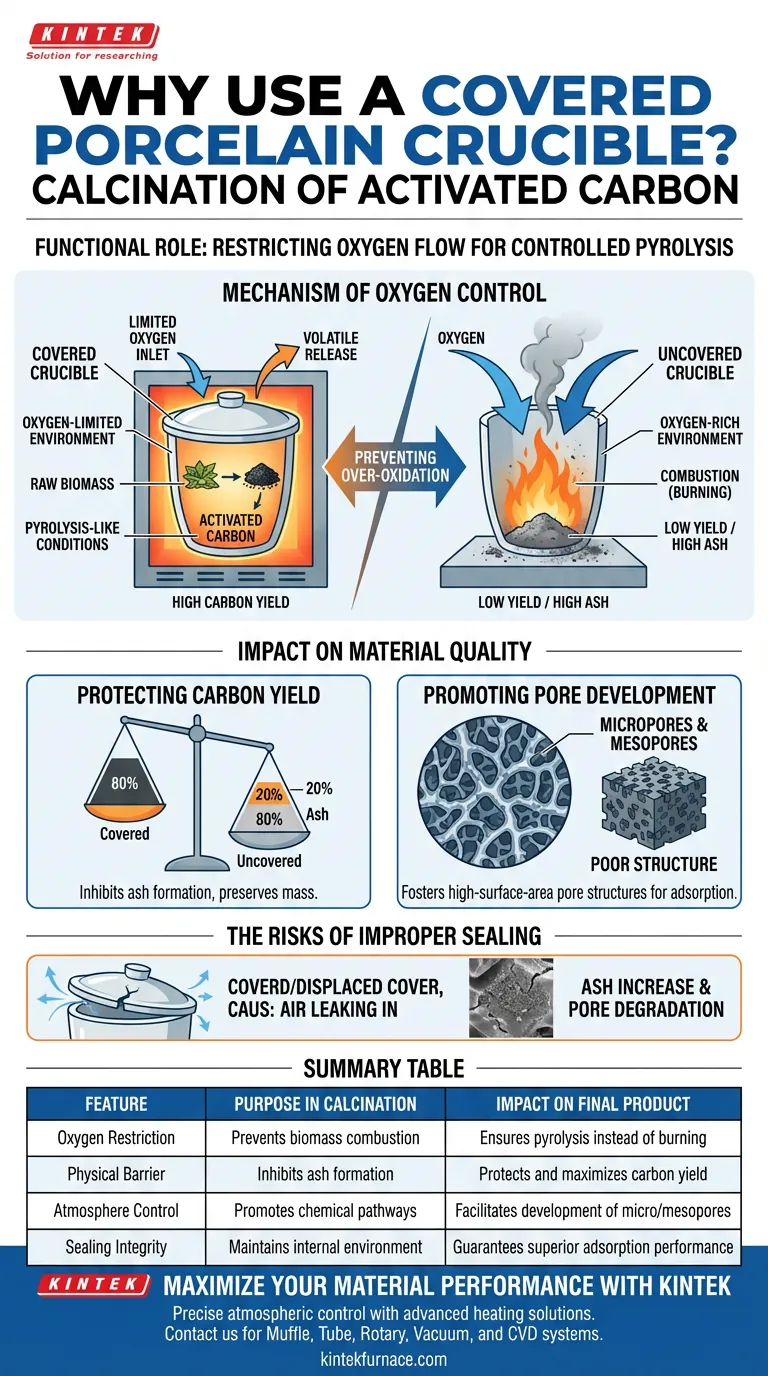
The use of a covered porcelain crucible is strictly functional: it serves as a physical barrier to restrict oxygen flow to the raw biomass during the heating process. By creating an oxygen-limited environment, the cover prevents the material from combusting completely, ensuring it undergoes a process similar to pyrolysis rather than simple burning.
By effectively limiting air intake, the cover creates a controlled environment that inhibits excessive ash production, protecting the carbon yield and facilitating the development of critical pore structures.

The Mechanism of Oxygen Control
Creating Pyrolysis-Like Conditions
The primary role of the cover is to simulate a pyrolysis environment within the crucible.
Without this barrier, the high temperatures of calcination would invite an influx of ambient air. The cover restricts this interaction, maintaining an atmosphere where thermal decomposition occurs in the absence of abundant oxygen.
Preventing Over-Oxidation
The greatest risk during calcination is over-oxidation.
If oxygen levels inside the crucible are unchecked, the biomass does not just carbonize; it burns. This reaction converts valuable organic material into useless ash, destroying the potential for activated carbon.
Impact on Material Quality
Protecting Carbon Yield
The economic and practical efficiency of the process depends on the yield of carbon.
By inhibiting the formation of ash caused by over-oxidation, the covered crucible ensures a higher percentage of the raw material is converted into usable carbon. This directly preserves the mass of the final product.
Promoting Pore Development
The physical structure of activated carbon is defined by its surface area and porosity.
The oxygen-limited environment fosters the specific chemical pathways required to form microporous and mesoporous structures. These pores are the active sites responsible for the material's adsorption capabilities.
The Risks of Improper Sealing
The Consequence of Air Leaks
While the crucible does not need to be hermetically sealed, a significant breach in the "physical barrier" defeats the purpose of the process.
If the cover is displaced or too loose, the internal environment shifts back toward combustion. This leads to a rapid increase in ash content and a degradation of the pore structure, rendering the activated carbon less effective for filtration or adsorption tasks.
Optimizing Your Calcination Strategy
To ensure you achieve the desired material properties, align your process with your specific objectives:
- If your primary focus is maximizing yield: Ensure the crucible cover is securely placed to minimize mass loss due to ash formation.
- If your primary focus is adsorption performance: strictly maintain the oxygen-limited environment to favor the development of high-surface-area micropores and mesopores.
Control the oxygen, and you control the quality of the carbon.
Summary Table:
| Feature | Purpose in Calcination | Impact on Final Product |
|---|---|---|
| Oxygen Restriction | Prevents biomass combustion | Ensures pyrolysis instead of burning |
| Physical Barrier | Inhibits ash formation | Protects and maximizes carbon yield |
| Atmosphere Control | Promotes chemical pathways | Facilitates development of micro/mesopores |
| Sealing Integrity | Maintains internal environment | Guarantees superior adsorption performance |
Maximize Your Material Performance with KINTEK
Precise atmospheric control is the difference between high-quality activated carbon and useless ash. KINTEK provides the advanced heating solutions you need to master your calcination process. Backed by expert R&D and manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, as well as other lab high-temperature furnaces—all fully customizable to meet your unique research or production needs.
Don't let improper oxygen control compromise your yield. Contact KINTEK today to discover how our high-precision equipment can enhance your laboratory’s efficiency and material quality!
Visual Guide
References
- Dzilal Amir, Nurul Sakinah Engliman. Investigating the synthesis parameters of durian skin-based activated carbon and the effects of silver nanocatalysts on its recyclability in methylene blue removal. DOI: 10.1186/s11671-024-03974-1
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the technical necessity of using a glass boat in a pyrolysis furnace? Precision in Thermal Decomposition
- Why are high-temperature ceramic crucibles used for chalcopyrite? Ensure Purity in Ore Thermal Treatment
- What is the primary function of a high-purity vacuum-sealed quartz tube in the Modified Bridgman technique? Key Role
- What process challenges are addressed by vacuum filtration equipment during the construction of CsPbBr3@CA-SiO2 films?
- What are the core functions of high-purity graphite molds and graphite paper in SPS? Optimize Sintering Quality
- What is the critical role of the vacuum filter in a waste magnesium vacuum distillation system? The Essential Protection for Your Vacuum Pump
- Why are high-purity MgO crucibles used for PbO oxidation? Essential Chemical Inertness for Master Slags
- How does a high-precision heating stage contribute to the drying and crystallization of FAPbBr3 nanosheets?


















