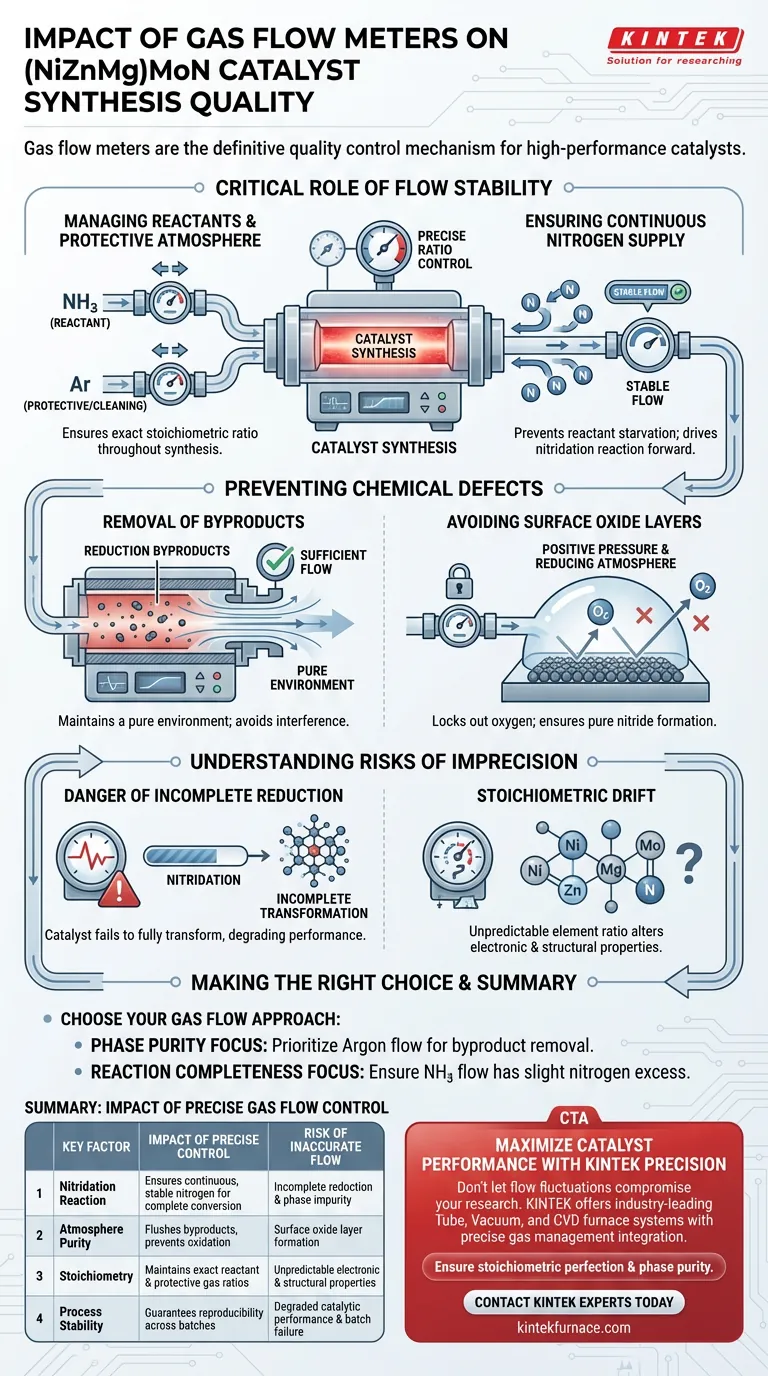
Gas flow meters act as the definitive quality control mechanism during the synthesis of (NiZnMg)MoN catalysts. By strictly regulating the intake of ammonia and argon into the tube furnace, these devices ensure the chemical environment remains stable enough to achieve the precise stoichiometric ratio required for a high-performance catalyst.
The stability provided by gas flow meters is the primary defense against structural defects. By maintaining a constant supply of nitrogen and flushing out byproducts, precise flow control prevents incomplete reduction and surface oxidation, ensuring the material achieves its intended chemical composition.
The Critical Role of Flow Stability
Managing Reactant and Protective Environments
In the nitridation process, gas flow meters govern two distinct streams: ammonia ($NH_3$) and argon ($Ar$).
Ammonia serves as the active reactant, while argon acts as a protective or cleaning gas.
Precise metering ensures that the ratio between the reactant and the protective atmosphere is maintained exactly as designed throughout the synthesis.
Ensuring Continuous Nitrogen Supply
A stable flow of ammonia is necessary to provide a consistent source of nitrogen.
This continuous supply is required to drive the nitridation reaction forward without interruption.
If the flow meter fails to maintain this supply, the synthesis becomes starved of reactants, compromising the final structure.
Preventing Chemical Defects
Removal of Reduction Byproducts
As the reaction proceeds, it generates reduction byproducts that can interfere with synthesis if allowed to accumulate.
Gas flow meters ensure the flow rate is sufficient to physically flush these byproducts out of the reaction zone in a timely manner.
This clearing action maintains a pure environment around the developing catalyst.
Avoiding Surface Oxide Layers
One of the most significant risks in this process is the formation of oxide layers on the catalyst surface.
Precise flow control prevents this by maintaining positive pressure and a reducing atmosphere, effectively locking out oxygen.
This ensures the final product is a pure nitride rather than a degraded oxide-nitride hybrid.
Understanding the Risks of Imprecision
The Danger of Incomplete Reduction
If gas flow fluctuates or drops below the required threshold, the reduction process will not complete.
This results in a catalyst that has failed to fully transform into the (NiZnMg)MoN phase.
Such incomplete reduction directly degrades the material's catalytic performance.
Stoichiometric Drift
The ultimate goal of using flow meters is to achieve a specific stoichiometric ratio of the elements involved.
Without the precision offered by these meters, the ratio of Nitrogen to the metal components (Ni, Zn, Mg, Mo) becomes unpredictable.
A deviation in this ratio alters the fundamental electronic and structural properties of the catalyst.
Making the Right Choice for Your Goal
To ensure the highest quality synthesis, your approach to gas flow control should align with your specific stability requirements.
- If your primary focus is Phase Purity: Prioritize the precision of the argon flow to effectively remove byproducts and prevent surface oxidation.
- If your primary focus is Reaction Completeness: Ensure the ammonia flow meter is calibrated to deliver a slight excess of nitrogen to prevent reactant starvation.
Mastering gas flow dynamics is the single most effective step to guaranteeing the reproducibility of your catalyst synthesis.
Summary Table:
| Key Factor | Impact of Precise Gas Flow Control | Risk of Inaccurate Flow |
|---|---|---|
| Nitridation Reaction | Ensures a continuous, stable nitrogen supply for complete conversion. | Incomplete reduction and phase impurity. |
| Atmosphere Purity | Effectively flushes out reduction byproducts and prevents oxidation. | Surface oxide layer formation. |
| Stoichiometry | Maintains exact ratios between NH3 reactants and Ar protective gases. | Unpredictable electronic and structural properties. |
| Process Stability | Guarantees reproducibility across multiple synthesis batches. | Degraded catalytic performance and batch failure. |
Maximize Your Catalyst Performance with KINTEK Precision
Don't let flow fluctuations compromise your material research. KINTEK provides industry-leading high-temperature lab furnaces—including specialized Tube, Vacuum, and CVD systems—engineered to integrate seamlessly with precise gas management tools.
Backed by expert R&D and world-class manufacturing, our systems are fully customizable to meet the rigorous demands of (NiZnMg)MoN synthesis and other advanced nitridation processes. Ensure stoichiometric perfection and phase purity in every batch.
Contact KINTEK Experts Today to Customize Your High-Temp Solution
Visual Guide
References
- (NiZnMg)MoN with optimized d-band center enables industrial-level hydrogen production. DOI: 10.1007/s40843-025-3462-6
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
- Stainless Steel KF ISO Vacuum Flange Blind Plate for High Vacuum Systems
- Ultra High Vacuum Observation Window Stainless Steel Flange Sapphire Glass Sight Glass for KF
- Ultra High Vacuum CF Observation Window Flange with High Borosilicate Glass Sight Glass
People Also Ask
- What is the function of a copper turning purification device? Enhance Your Sintering Furnace Gas Purity
- Why are high-purity alumina crucibles required for high-temperature melting studies of sintering ores? Expert Insights
- What is the role of an optical pyrometer in diffusion bonding? Ensure Precision in High-Temperature Simulations
- What roles do ceramic crucibles play in 500 °C pre-calcination? Ensure Pure Layered Oxide Synthesis
- Why use sealed quartz tubes & vacuum for Mg-Zn/Mg-Cd alloy prep? Ensure Compositional Purity
- What are the primary functions of the vacuum pump system and inert gases? Achieve High-Purity Atomization
- What is the purpose of configuring a hot gas filter within a Catalytic Hydropyrolysis (CHP) process? Ensure Reactor Life
- How do a three-stub tuner and a sliding short contribute to microwave carbothermic reduction? Maximize Energy Efficiency













