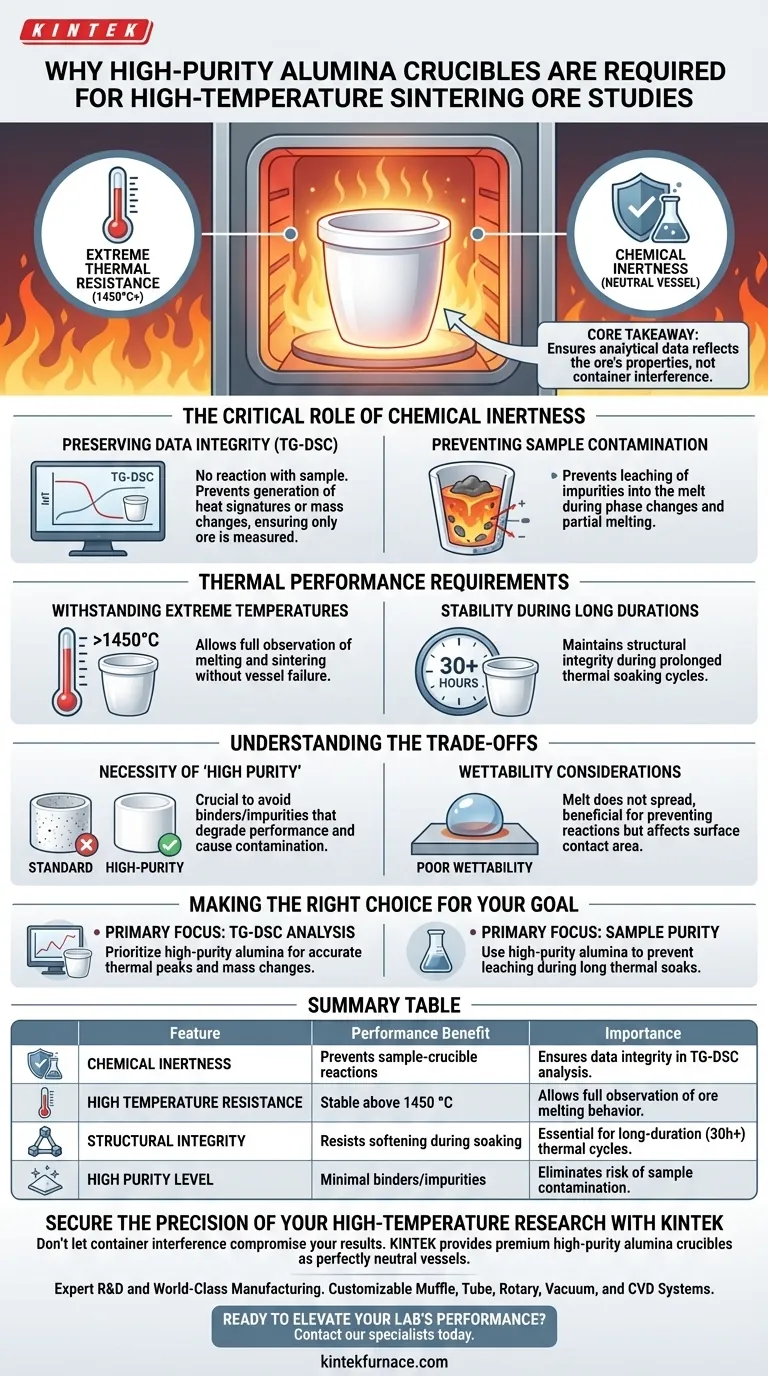
High-purity alumina crucibles are strictly required for high-temperature melting studies of sintering ores because of their dual capacity for extreme thermal resistance and chemical inertness. They can withstand temperatures exceeding 1450 °C without reacting with mineral samples, which is essential for ensuring that analytical data reflects the properties of the ore rather than interference from the container.
Core Takeaway The validity of high-temperature ore analysis relies on the isolation of the sample from its environment. High-purity alumina provides a neutral vessel that prevents chemical cross-talk, ensuring that measured heat changes and mass variations are exclusively attributable to the sintering material itself.

The Critical Role of Chemical Inertness
Preserving Data Integrity
In techniques like Thermogravimetric-Differential Scanning Calorimetry (TG-DSC), the goal is to measure precise heat changes within the ore.
If the crucible reacts with the sample, it generates its own heat signatures or mass changes. High-purity alumina is chemically inert, meaning it does not participate in the reaction, effectively "disappearing" from the data set so only the ore is measured.
Preventing Sample Contamination
Sintering ores undergo complex phase changes and partial melting.
During this vulnerable state, a reactive container could leach impurities into the melt. Alumina prevents this contamination, ensuring the chemical composition of the final sintered product remains accurate to the original sample.
Thermal Performance Requirements
Withstanding Extreme Temperatures
Sintering studies frequently require temperatures that destroy standard labware.
High-purity alumina allows for experimentation at temperatures exceeding 1450 °C. This high ceiling is necessary to fully observe the melting and sintering behaviors of the ore without the risk of the vessel softening or failing.
Stability During Long Durations
Sintering often involves prolonged exposure to heat (thermal soaking).
Alumina maintains its structural integrity over long periods, such as 30-hour thermal soaking cycles. This stability ensures the experiment can run to completion without the mechanical failure of the crucible.
Understanding the Trade-offs
The Necessity of "High Purity"
Not all alumina is created equal; standard alumina may contain binders or impurities that degrade performance.
You must specifically utilize high-purity formulations to achieve the inertness described here. Lower-grade alumina may introduce the very contaminants you are trying to avoid, compromising the "neutral vessel" requirement.
Wettability Considerations
While generally advantageous, alumina exhibits poor wettability with many melts.
This is a benefit for preventing reactions, but it means the melt will not spread across the crucible surface. Researchers must account for this physical behavior when designing experiments where surface contact area is a variable.
Making the Right Choice for Your Goal
When selecting crucibles for metallurgical or mineralogical studies, align your choice with your specific analytical needs.
- If your primary focus is TG-DSC Analysis: Prioritize high-purity alumina to ensure that all recorded thermal peaks and mass changes are exclusively from the sintering ore.
- If your primary focus is Sample Purity: Use high-purity alumina to prevent the leaching of container elements into the melt during long thermal soaks.
By selecting high-purity alumina, you transform the variable of the container into a constant, securing the scientific validity of your high-temperature results.
Summary Table:
| Feature | Performance Benefit | Importance in Sintering Studies |
|---|---|---|
| Chemical Inertness | Prevents sample-crucible reactions | Ensures data integrity in TG-DSC analysis |
| High Temperature Resistance | Stable above 1450 °C | Allows full observation of ore melting behavior |
| Structural Integrity | Resists softening during soaking | Essential for long-duration (30h+) thermal cycles |
| High Purity Level | Minimal binders/impurities | Eliminates risk of sample contamination |
Secure the Precision of Your High-Temperature Research with KINTEK
Don't let container interference compromise your analytical results. KINTEK provides premium high-purity alumina crucibles designed to act as perfectly neutral vessels for your most demanding sintering and melting studies.
Backed by expert R&D and world-class manufacturing, we offer a full range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all of which can be customized to your lab's unique high-temperature needs. Whether you are performing TG-DSC analysis or metallurgical thermal soaking, our equipment ensures that your data reflects your material, not your tools.
Ready to elevate your lab's performance? Contact our specialists today to discuss your custom thermal processing requirements!
Visual Guide
References
- Seong‐Jin Kim, Sung‐Mo Jung. Effect of Mill-Scale and Calcined Dolomite on High Al2O3 Sinter and Its Phase Development. DOI: 10.1007/s11663-025-03677-7
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Magnesium Extraction and Purification Condensing Tube Furnace
People Also Ask
- What roles do high-purity graphite molds play during the Spark Plasma Sintering (SPS) of Ba0.95La0.05FeO3-δ? Essential Guide
- What is the primary role of laboratory furnaces in manufacturing and scientific processes? Unlock Precision Thermal Control
- What is the critical physical function of a laboratory electric blast drying oven in phosphor gel treatment?
- What is the function of the nitrogen environment in pyrolysis? Mastering Carbonization with Laboratory Furnaces
- Why is a graphite crucible used and the melt temperature maintained at 750°C for AA7150-Al2O3? Optimize Your Composite
- What is the significance of using ceramic or quartz sample boats for solid fuels? Ensure Precise Thermal Analysis
- What are the technical advantages of using high-purity quartz tubes? Optimize Heat and Purity in Combustion Analysis
- What are the technical advantages of using quartz tubes for fiber optic sensors? Optimize High-Temp Performance



















