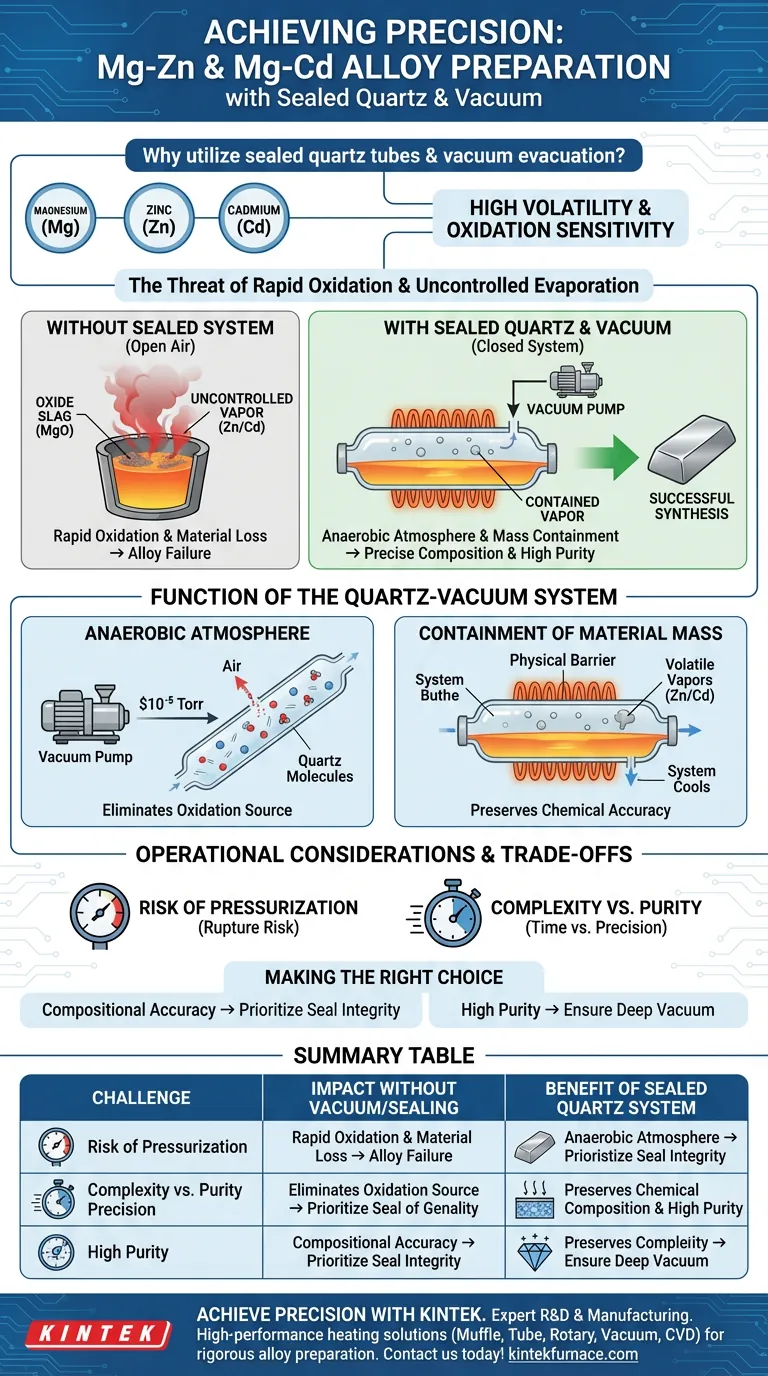
The necessity stems from the specific physical properties of magnesium, zinc, and cadmium. These metals are characterized by high volatility and extreme sensitivity to oxidation at elevated temperatures. Utilizing sealed quartz tubes with vacuum evacuation creates an isolated environment that prevents the raw materials from burning up or evaporating away, ensuring the final alloy matches your intended chemical composition.
Core Takeaway This technique solves two critical failure points: it eliminates atmospheric oxygen to prevent oxide formation and creates a closed system to contain the high vapor pressure of volatile elements, guaranteeing compositional precision.
The Chemical Vulnerability of Raw Materials
The Threat of Rapid Oxidation
Magnesium is highly reactive. When exposed to oxygen at melting temperatures, it rapidly forms magnesium oxide.
This oxidation is not merely a surface issue; it can degrade a significant portion of the raw material into useless slag. Without a protective environment, the introduction of oxides compromises the structural integrity and purity of the resulting alloy.
Managing High Vapor Pressure
Zinc and cadmium possess high vapor pressures, meaning they have a strong tendency to turn into gas at relatively low temperatures.
In an open system, these elements would suffer from uncontrolled evaporation during the melting process. This results in a significant loss of material mass, making it impossible to predict or control the ratio of elements in the final alloy.
Function of the Quartz-Vacuum System
Establishing an Anaerobic Atmosphere
The primary role of the vacuum evacuation is to remove air from the reaction vessel before heating begins.
By reducing internal pressure (often to levels such as $10^{-5}$ Torr), you create an anaerobic protective atmosphere. This effectively eliminates the source of oxidation, ensuring that the magnesium remains metallic and pure throughout the heating cycle.
Containment of Material Mass
The sealed quartz tube acts as a physical barrier against material loss.
While some evaporation of zinc or cadmium is inevitable inside the tube, the closed system ensures this vapor cannot escape. As the system cools or reaches equilibrium, these vapors are retained within the alloy matrix rather than being lost to the environment, preserving the accuracy of the chemical composition.
Operational Considerations and Trade-offs
The Risk of Pressurization
While sealing is necessary, it introduces the risk of internal pressure buildup.
As the volatile metals heat up and vaporize, the pressure inside the quartz tube increases. If the tube is weak or the temperature ramp is too aggressive, there is a risk of rupture.
Complexity vs. Purity
Using vacuum-sealed quartz tubes adds significant time and complexity to the preparation process compared to open-air melting.
However, this is a necessary trade-off. Conventional methods like muffle furnace heating without encapsulation would result in severe oxidation and compositional drift, rendering the synthesis of precision Mg-Zn or Mg-Cd alloys impossible.
Making the Right Choice for Your Goal
To ensure the success of your alloy preparation, align your process with your specific requirements:
- If your primary focus is Compositional Accuracy: Prioritize the integrity of the seal; a leak-proof tube is the only way to prevent the loss of volatile Zinc or Cadmium vapors.
- If your primary focus is High Purity: Ensure you achieve a deep vacuum (pre-evacuation) to remove all traces of oxygen and moisture before sealing the tube.
By controlling the atmosphere and the containment, you transform a volatile chemical reaction into a precise metallurgical process.
Summary Table:
| Challenge | Impact without Vacuum/Sealing | Benefit of Sealed Quartz System |
|---|---|---|
| Oxidation | Rapid formation of MgO slag; material degradation | Anaerobic atmosphere prevents oxide formation |
| Volatility | Zinc/Cadmium vaporize and escape in open air | Closed system contains vapors to preserve mass |
| Purity | Introduction of atmospheric contaminants | Controlled environment ensures high chemical purity |
| Composition | Unpredictable element ratios due to mass loss | Precise control over the final alloy stoichiometry |
Achieve Precision in Volatile Alloy Synthesis with KINTEK
Don't let oxidation and material loss compromise your research. Backed by expert R&D and manufacturing, KINTEK provides high-performance heating solutions including Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet the rigorous demands of magnesium-zinc and magnesium-cadmium alloy preparation.
Our advanced furnace technologies provide the stable thermal environments and vacuum integration necessary for handling high vapor pressure materials with confidence.
Ready to elevate your metallurgical process? Contact us today to discuss your unique laboratory needs!
Visual Guide
References
- В. Н. Володин, Alexey Trebukhov. On the Problem of the Distillation Separation of Secondary Alloys of Magnesium with Zinc and Magnesium with Cadmium. DOI: 10.3390/met14060671
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- Why are support frames important for the alumina furnace tube? Prevent High-Temperature Deformation and Failure
- How do high-precision mass flow controllers (MFC) aid iron oxide reduction studies? Get Accurate Kinetic Data
- What is the function of an enhanced hydrothermal reactor with magnetic stirring? Optimize MoS2/C Synthesis Yield
- What are the technical considerations for selecting a stainless steel cylindrical vessel? Magnesium Test Chamber Guide
- What role do quartz tubes play in semiconductor manufacturing? Essential for Purity and High-Temp Processes
- What are the benefits of 150mm thick ceramic fiber blankets in furnaces? Boost Efficiency and Safety
- Why is zirconia grinding media preferred for NN-10ST ceramic powders? Ensure Purity & Dielectric Performance
- Why is a high vacuum pumping system necessary for carbon nanotube peapods? Achieve Precise Molecular Encapsulation



















