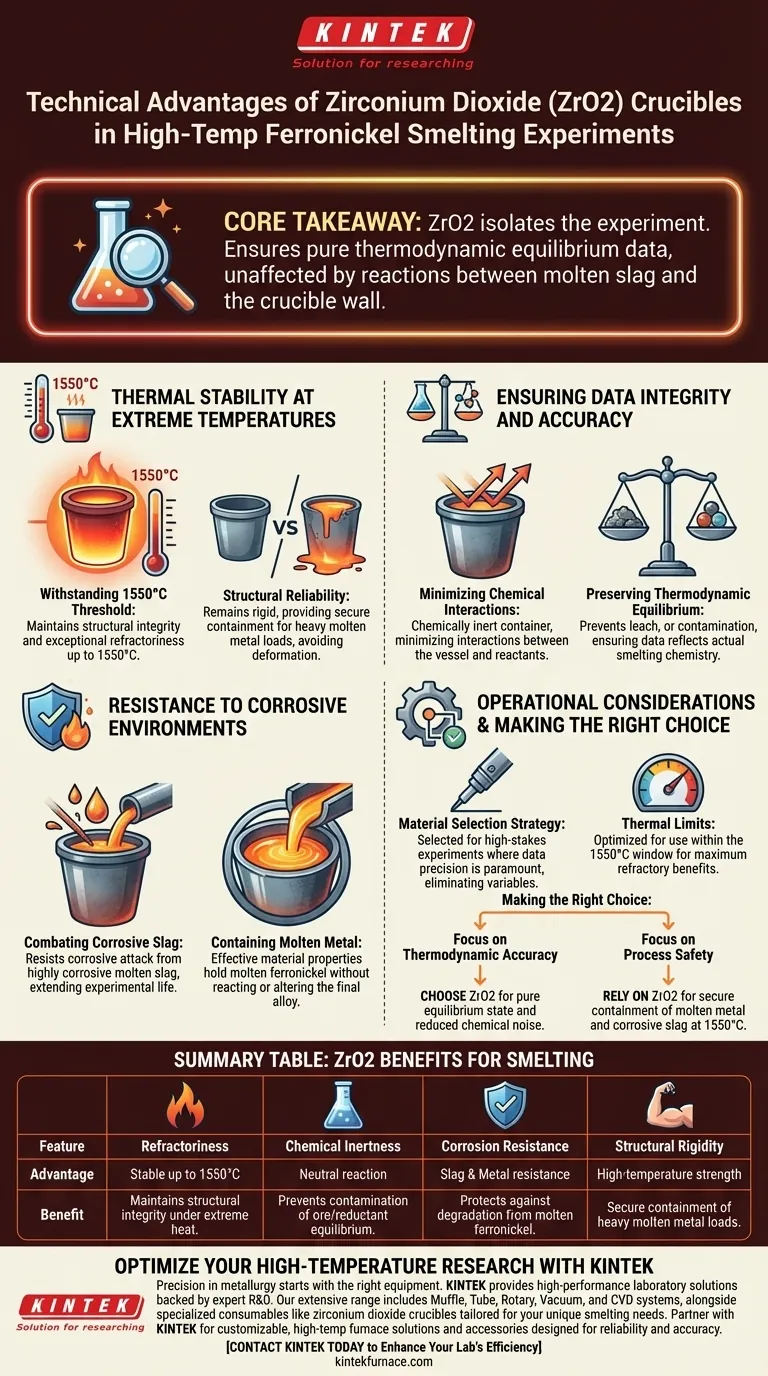
Zirconium dioxide (ZrO2) crucibles are the definitive choice for high-temperature ferronickel smelting experiments primarily due to their exceptional thermal stability and chemical inertness. By resisting degradation at temperatures reaching 1550°C, these vessels minimize interference from container materials, ensuring that experimental observations focus solely on the interactions between the ore and the reductant.
Core Takeaway The value of zirconium dioxide lies in its ability to isolate the experiment from the equipment. It ensures that thermodynamic equilibrium data remains pure and unadulterated by chemical reactions between the molten slag and the crucible wall.

Thermal Stability at Extreme Temperatures
Withstanding the 1550°C Threshold
Ferronickel smelting requires significant thermal energy to achieve the necessary phase changes. Zirconium dioxide crucibles exhibit exceptional refractoriness, maintaining structural integrity up to 1550°C.
Structural Reliability
At these elevated temperatures, many standard crucible materials would soften or deform. ZrO2 remains rigid, providing a secure containment vessel for the heavy molten metal load throughout the duration of the experiment.
Ensuring Data Integrity and Accuracy
Minimizing Chemical Interactions
In experimental metallurgy, the crucible must act as a neutral container, not a participant. Zirconium dioxide is chemically inert in this context, minimizing interactions between the vessel material and the reactants.
Preserving Thermodynamic Equilibrium
Accurate research depends on establishing a true thermodynamic equilibrium between the ore and the reductant. Because ZrO2 prevents leaching or contamination, the resulting data reflects the actual chemistry of the smelt rather than artifacts of crucible degradation.
Resistance to Corrosive Environments
Combating Corrosive Slag
Molten slag produced during smelting is highly corrosive and can rapidly eat through inferior materials. Zirconium dioxide is specifically noted for its resistance to this corrosive attack, extending the life of the experimental setup.
Containing Molten Metal
Beyond the slag, the molten ferronickel itself presents a containment challenge. The crucible’s material properties effectively hold the molten metal without reacting with it, ensuring the final alloy composition is not altered by the vessel.
Operational Considerations
Material Selection Strategy
While ZrO2 is "ideal" for these conditions, it is selected specifically for high-stakes experiments where data precision is paramount. Using lesser materials would introduce variables that could render thermodynamic calculations useless.
Thermal Limits
It is critical to note the operational ceiling mentioned is 1550°C. While robust, the material is optimized for this specific high-temperature window and should be utilized within these parameters to maintain its refractory benefits.
Making the Right Choice for Your Goal
When designing your ferronickel smelting experiments, use zirconium dioxide based on your specific data requirements:
- If your primary focus is Thermodynamic Accuracy: Choose ZrO2 to eliminate chemical noise and ensure the equilibrium state reflects only the ore and reductant.
- If your primary focus is Process Safety: Rely on ZrO2 for its refractory capability to securely contain molten metal and corrosive slag at 1550°C without structural failure.
By selecting zirconium dioxide, you convert the crucible from a potential variable into a reliable constant.
Summary Table:
| Feature | Advantage | Benefit to Smelting Experiments |
|---|---|---|
| Refractoriness | Stable up to 1550°C | Maintains structural integrity under extreme heat |
| Chemical Inertness | Neutral reaction | Prevents contamination of ore/reductant equilibrium |
| Corrosion Resistance | Slag & Metal resistance | Protects against degradation from molten ferronickel |
| Structural Rigidity | High-temperature strength | Secure containment of heavy molten metal loads |
Optimize Your High-Temperature Research with KINTEK
Precision in metallurgy starts with the right equipment. KINTEK provides high-performance laboratory solutions backed by expert R&D and manufacturing. Our extensive range includes Muffle, Tube, Rotary, Vacuum, and CVD systems, alongside specialized consumables like zirconium dioxide crucibles tailored for your unique smelting needs.
Don't let equipment variables compromise your thermodynamic data. Partner with KINTEK for customizable, high-temp furnace solutions and accessories designed for reliability and accuracy.
Contact KINTEK Today to Enhance Your Lab’s Efficiency
Visual Guide
References
- Erdenebold Urtnasan, Jei‐Pil Wang. Relationship Between Thermodynamic Modeling and Experimental Process for Optimization Ferro-Nickel Smelting. DOI: 10.3390/min15020101
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- Why is graphite foil used to line graphite molds before loading titanium alloy powder? Ensure Purity and Protect Molds
- What is the importance of using a Mass Flow Controller (MFC)? Enhance Molybdenum Phosphide (MoP) Synthesis Precision
- What role does a quartz substrate holder play in MoS2 growth? Optimize Thin Film Deposition with Precision Hardware
- How does a water circulating vacuum pump create negative pressure? Discover the Liquid-Ring Mechanism for Efficient Lab Vacuum
- What is the primary purpose of a benchtop blast drying oven? Optimize Barium Titanate Ceramic Preparation
- How is measurement accuracy maintained for infrared pyrometers? Master Optical Hygiene for High-Temp Metallic Melts
- Why are flexible graphite gaskets utilized for sealing in LiF-BeF2 molten salt experiments? High-Resilience Solutions
- How does the choice of high-purity ceramic crucibles impact glass phantoms? Unlock Optical Precision in Sintering



















