The primary function of a benchtop blast drying oven is to efficiently facilitate the solvent evaporation of Barium Titanate slurries immediately following the ball milling process. By utilizing forced convection circulation, this equipment achieves rapid drying at controlled low temperatures, ensuring the transition from wet slurry to dry powder occurs without compromising the material's physical structure.
While the immediate mechanism is dehydration, the critical outcome is the preservation of powder morphology. This method is specifically employed to minimize particle clumping, ensuring the final material possesses the bulk density and flow characteristics required for high-quality ceramic molding.
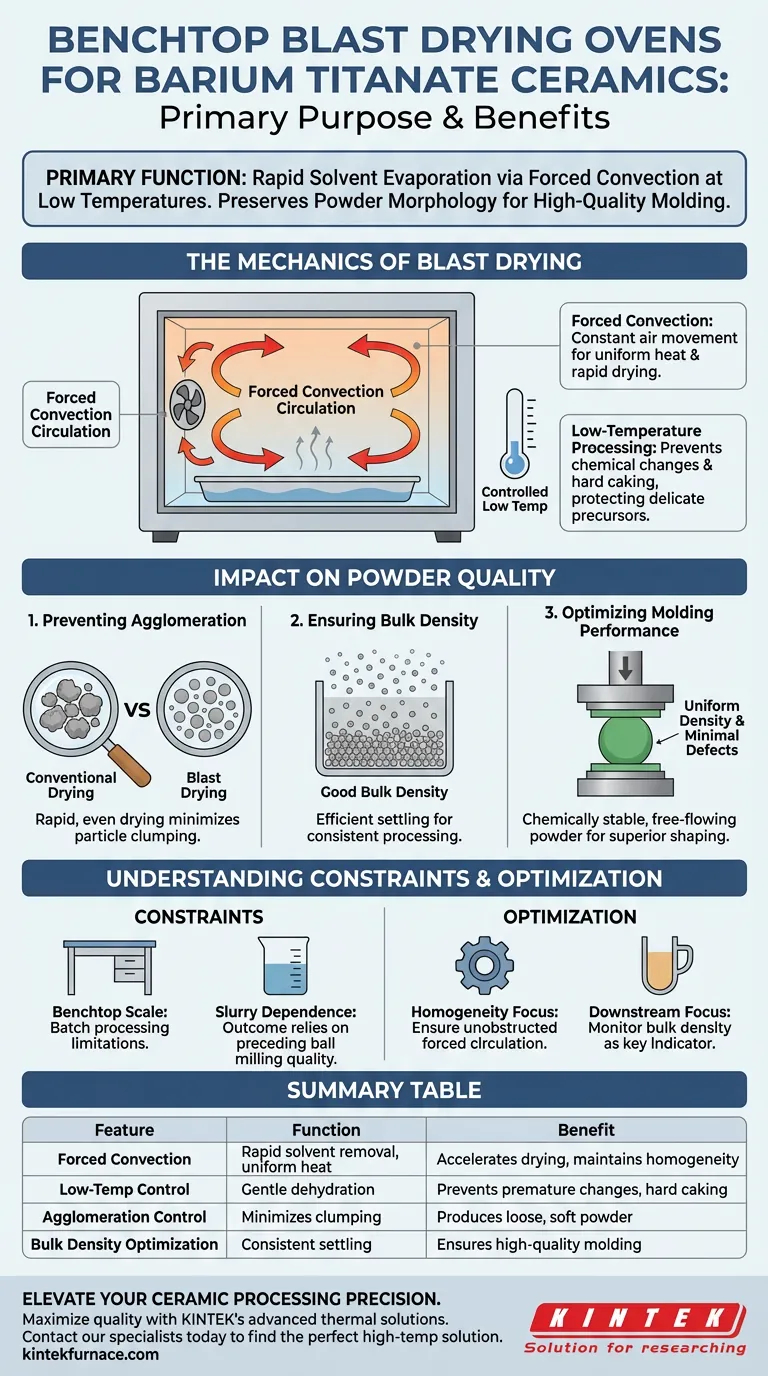
The Mechanics of Blast Drying
Forced Convection Circulation
The core advantage of this equipment lies in its air circulation system. Unlike static ovens that rely on passive heat transfer, a blast drying oven uses forced convection to circulate heated air throughout the chamber.
This constant movement ensures uniform temperature distribution. It rapidly carries away evaporated solvent vapor, significantly accelerating the drying rate compared to conventional methods.
Low-Temperature Processing
The process is designed to operate at low temperatures despite the rapid drying speed. Maintaining a lower thermal profile is essential for delicate ceramic precursors.
High heat can induce premature chemical changes or hard caking. By keeping the temperature moderate, the oven removes the solvent while leaving the chemical composition of the Barium Titanate intact.
Impact on Powder Quality
Preventing Agglomeration
One of the most persistent challenges in ceramic preparation is the tendency of powders to clump together as they dry. Significant agglomeration creates hard clusters that are difficult to break down later.
The blast drying oven addresses this by drying the slurry quickly and evenly. This rapid solvent removal prevents the formation of strong inter-particle bridges, resulting in a looser, softer powder structure.
Ensuring Bulk Density
The physical state of the dried powder dictates how well it packs together. The reference establishes that this drying method ensures good bulk density.
Proper bulk density is a critical metric. It implies that the powder particles settle efficiently, which is a prerequisite for consistency in subsequent processing steps.
Optimizing Molding Performance
The ultimate goal of the drying phase is to prepare the material for shaping. The output of the blast drying oven is specifically noted for having optimal molding performance.
Because the powder is chemically stable and free of hard agglomerates, it can be pressed or formed into green bodies with uniform density and minimal defects.
Understanding the Constraints
Scale and Throughput
The specific classification of this equipment as a "benchtop" unit implies a limitation regarding production volume. While highly effective for laboratory scale or small-batch preparation, it operates on a batch-processing model rather than a continuous stream.
Dependence on Slurry Consistency
While the oven manages evaporation, the quality of the final dry powder is still heavily dependent on the preceding ball milling step. The oven can preserve the dispersion achieved during milling, but it cannot correct a slurry that was poorly mixed or stabilized beforehand.
Optimizing Your Ceramic Preparation
To get the most out of a benchtop blast drying oven, align your usage with your specific processing goals.
- If your primary focus is Powder Homogeneity: Ensure the forced circulation is active and unobstructed to prevent "dead spots" where uneven drying could lead to localized agglomeration.
- If your primary focus is Downstream Processing: Monitor the bulk density of the dried output carefully, as this is your best indicator of how well the powder will behave during the molding phase.
By controlling the evaporation rate and temperature, you transform a simple drying step into a critical quality control measure for your Barium Titanate ceramics.
Summary Table:
| Feature | Function in Barium Titanate Prep | Benefit to Final Material |
|---|---|---|
| Forced Convection | Rapid solvent removal & uniform heat | Accelerates drying while maintaining homogeneity |
| Low-Temp Control | Gentle dehydration of slurry | Prevents premature chemical changes and hard caking |
| Agglomeration Control | Minimizes particle clumping | Produces loose, soft powder for better dispersion |
| Bulk Density Optimization | Consistent particle settling | Ensures high-quality molding and shaping performance |
Elevate Your Ceramic Processing Precision
Maximize your material quality with KINTEK’s advanced thermal solutions. Backed by expert R&D and manufacturing, KINTEK offers a wide range of Muffle, Tube, Rotary, Vacuum, and CVD systems, along with specialized lab high-temp furnaces tailored for precise drying and sintering. Whether you are optimizing Barium Titanate morphology or scaling up production, our customizable systems deliver the uniformity and control your research demands.
Ready to refine your laboratory workflow? Contact our specialists today to find the perfect high-temp solution for your unique needs.
Visual Guide
References
- Effect of Beam Power on Intermetallic Compound Formation of Electron Beam-Welded Cu and Al6082-T6 Dissimilar Joints. DOI: 10.3390/eng6010006
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What should be evaluated when assessing supplier reliability for alumina ceramic furnace tubes? Ensure Consistent Performance and Support
- What factors influence the lifespan of alumina ceramic furnace tubes? Maximize Durability and Performance
- Why are a press and pelletizing molds necessary when preparing pellets for magnesium smelting? Ensure Smelting Efficiency and Control
- What are some key terms related to Laboratory Furnaces? Demystify Types Like Muffle and Tube Furnaces
- What role does a precision drying oven play in the pre-treatment of Bi-Fe oxide powders? Safeguard Your Nano-Morphology
- What roles do metal shielding disks and heat shields play in in-situ SEM? Ensure Precision & Protect Your Lab Equipment
- What characteristics are required for reaction vessels in PI-COFs synthesis? Ensure High-Pressure Safety and Purity
- Why are high-purity quartz tubes and quartz boats preferred for plastic pyrolysis? Ensure Precise, Pure Results



















