High-purity quartz dominates plastic pyrolysis because it serves as a chemically invisible shield between your sample and the harsh environment of the furnace. It is preferred because it withstands temperatures up to 1200°C without degrading, while its chemical inertness prevents the reaction vessel from contaminating the resulting pyrolysis oil or gases.
Core Takeaway Pyrolysis is a chemically aggressive process where standard materials often fail or alter results. Quartz is the industry standard not merely for its heat resistance, but for its neutrality—it ensures that your experimental data reflects the plastic's true composition, undistorted by reactions with the container walls.
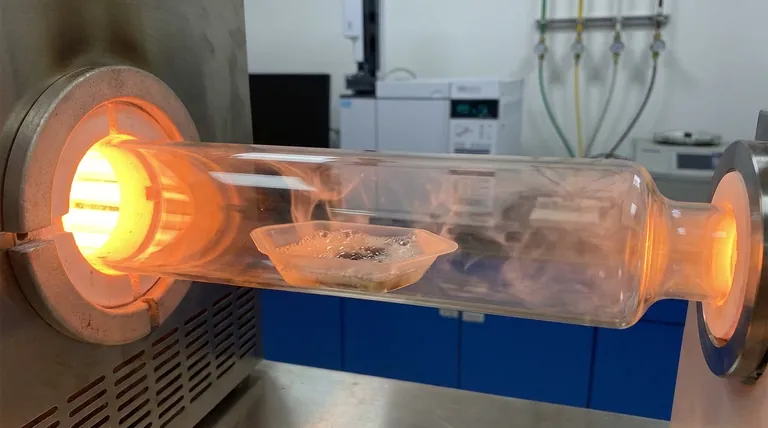
The Imperative of Chemical Inertness
Preventing Catalytic Side Reactions
At temperatures exceeding 1000 K, molten plastics and the gases they release become highly reactive and corrosive.
Standard metal containers can react with these substances, potentially acting as unintended catalysts. This alters the chemical breakdown process.
High-purity quartz boats and tubes remain chemically inert. They do not react with the melt or the corrosive vapors, ensuring the reaction proceeds exactly as intended.
Protecting Analytical Accuracy
The primary goal of many pyrolysis experiments is to analyze the exact composition of the resulting products, such as pyrolysis oil.
If the container material degrades or leaches into the sample, the analytical results are compromised.
Quartz protects the purity of the end products. This guarantees that the data collected is objective, accurate, and reproducible.
Thermal Resilience and Stability
Surviving Extreme Temperatures
Plastic pyrolysis requires intense energy to break chemical bonds.
High-purity quartz offers exceptional high-temperature resistance, capable of withstanding environments up to 1200°C.
This creates a safety margin for researchers working at the upper limits of standard pyrolysis protocols.
Withstanding Thermal Shock
Experimental cycles often involve rapid heating or cooling phases.
Materials with high thermal expansion rates can crack or shatter under these shifts.
Quartz possesses superior thermal shock stability, allowing it to endure rapid temperature changes without structural failure.
Process Visibility and Control
Real-Time Observation
Unlike opaque ceramic or metal vessels, quartz is transparent.
This allows researchers to visually monitor the physical state of the reactants in real-time.
You can observe critical transitions, such as melting, bubbling, and char formation, providing qualitative data that sensors might miss.
Maintaining Anaerobic Conditions
Pyrolysis is defined by the absence of oxygen.
Quartz tubes offer excellent sealing capabilities.
This allows for the maintenance of a strict anaerobic environment, preventing oxidation that would ruin the experiment.
Understanding the Trade-offs
Mechanical Fragility
While quartz is thermally robust, it is mechanically brittle.
It does not tolerate physical impact or bending stress well. Improper handling during loading or clamping can easily result in shattered tubes.
Cost Implications
High-purity quartz is significantly more expensive than borosilicate glass or standard ceramics.
It represents an investment in data quality rather than a low-cost consumable.
Making the Right Choice for Your Experiment
To maximize the success of your pyrolysis project, align your material choice with your specific analytical goals:
- If your primary focus is Analytical Precision: Choose quartz to eliminate the risk of heavy metal contamination or catalytic effects from metallic vessels.
- If your primary focus is Process Monitoring: Choose quartz to leverage its transparency for visual confirmation of reaction phases and char buildup.
- If your primary focus is High-Temperature Safety: Choose quartz if your protocol demands temperatures approaching 1200°C, where borosilicate glass would soften or melt.
Select high-purity quartz when the integrity of your data is more critical than the cost of your consumables.
Summary Table:
| Feature | High-Purity Quartz | Standard Metal/Ceramic |
|---|---|---|
| Temperature Limit | Up to 1200°C | Varies (Risk of degradation) |
| Chemical Inertness | Excellent (No catalytic interference) | Low (Potential side reactions) |
| Thermal Shock Resistance | Superior | Moderate to High |
| Visibility | Transparent (Real-time monitoring) | Opaque |
| Best Use Case | Analytical precision & purity | Basic heating applications |
Elevate Your Pyrolysis Research with KINTEK Precision
Don't let container contamination compromise your analytical data. KINTEK provides high-performance high-purity quartz components designed to withstand the rigors of aggressive chemical environments.
Backed by expert R&D and world-class manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, along with other lab high-temperature furnaces—all fully customizable to meet your unique experimental needs. Whether you are scaling up plastic recycling research or conducting high-purity material synthesis, our technical team is ready to support your goals with reliable, high-quality solutions.
Ready to optimize your lab's thermal processes? Contact us today to discuss your custom furnace needs!
References
- Yong Li, Fengfu Yin. Synergistic Effects Between Mixed Plastics and Their Impact on Pyrolysis Behavior and Pyrolysis Products. DOI: 10.3390/molecules29246059
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant Rotating Furnace
People Also Ask
- What is the function of high-precision molds and laboratory presses in LLTO preparation? Ensure Material Consistency
- What is the importance of using spot-welded K-type thermocouples in DP steel heat treatment? Master Thermal Precision
- How are laboratory vacuum pumps utilized in 1T-TaS2 crystal preparation? Ensure Peak Sample Purity
- What should be evaluated when assessing supplier reliability for alumina ceramic furnace tubes? Ensure Consistent Performance and Support
- Why are zirconia grinding jars and milling balls ideal for Bismuth Telluride? Achieve 200nm Purity and Performance
- Why is a laboratory reactor necessary for modified phenolic resin synthesis? Achieve Precision in Polymerization
- What is the function of a vacuum rotary vane pump in hydrogen measurement? Ensure High-Purity Gas Analysis Baseline
- How does the optical clarity of quartz tubes benefit laboratory processes? Enhance Control and Accuracy in High-Temp Experiments



















