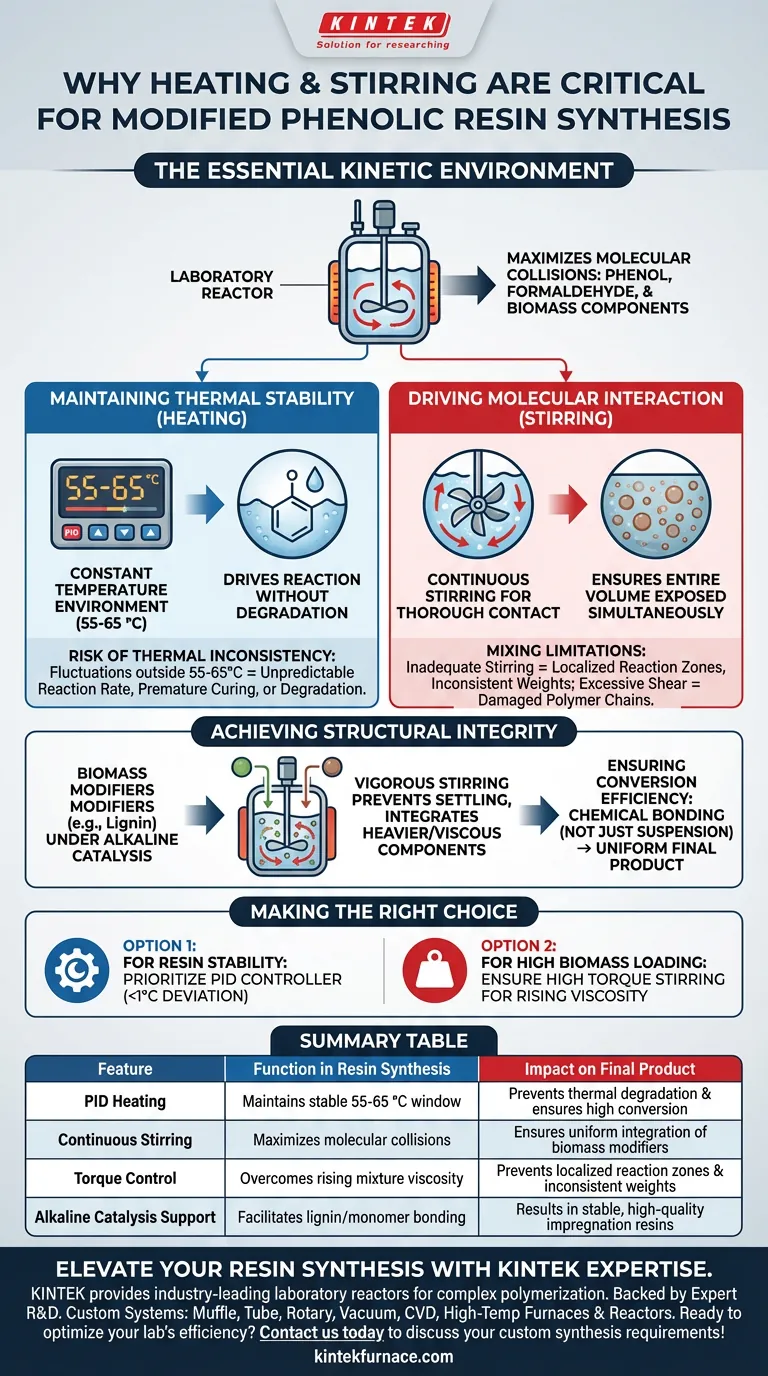
A laboratory reactor equipped with heating and stirring functions is strictly necessary for the synthesis of modified phenolic resins because it creates the specific kinetic environment required for complex polymerization. Without precise thermal regulation and mechanical agitation, it is impossible to achieve the uniform molecular interactions required to integrate modifiers effectively.
The reactor’s primary function is to maximize molecular collisions between phenol, formaldehyde, and biomass components, guaranteeing the high conversion rates necessary for forming stable, high-quality impregnation resins.

The Mechanics of Successful Polymerization
Maintaining Thermal Stability
The synthesis of modified phenolic resins relies on a specific condensation reaction that is highly sensitive to temperature.
To drive this reaction forward without degrading the materials, the reactor must provide a constant temperature environment.
The optimal window for this process is typically between 55 and 65 °C.
Driving Molecular Interaction
Heating alone is insufficient; the reactants must physically meet to bond.
Continuous stirring is essential to ensure thorough contact between the primary chemical reactants (phenol and formaldehyde) and the modifier (biomass monomers).
This mechanical action ensures that the entire volume of the mixture is exposed to the same reaction conditions simultaneously.
Achieving Structural Integrity
Integrating Biomass Modifiers
Modified phenolic resins often incorporate biomass components, such as lignin, under alkaline catalysis.
These modifiers can be difficult to disperse compared to pure liquid chemicals.
Vigorous stirring ensures these heavier or more viscous components do not settle, allowing them to be successfully integrated into the resin molecular chain.
Ensuring Conversion Efficiency
The ultimate goal of the synthesis is a stable impregnation resin with a high conversion rate.
If the reaction environment varies locally—due to cold spots or poor mixing—the conversion will be incomplete.
The reactor guarantees that the biomass components are chemically bonded, rather than just physically suspended, resulting in a uniform final product.
Understanding the Trade-offs
The Risk of Thermal Inconsistency
While heating is vital, "more heat" is not better; precision is key.
If the temperature fluctuates significantly outside the 55-65 °C range, the reaction rate becomes unpredictable.
Exceeding the temperature limit can lead to premature curing or degradation of the biomass, while falling below it results in an incomplete reaction.
Mixing Limitations
Stirring speed must be balanced against the viscosity of the resin.
Inadequate stirring leads to localized reaction zones, resulting in a resin with inconsistent molecular weights.
Conversely, excessive shear force in later stages of polymerization (as viscosity rises) can potentially damage the polymer chains or overheat the mixture via friction.
Making the Right Choice for Your Goal
To ensure your synthesis yields a usable modified phenolic resin, align your equipment settings with your specific objectives:
- If your primary focus is Resin Stability: Prioritize a reactor with a PID temperature controller to maintain the 55-65 °C range with less than 1°C deviation.
- If your primary focus is High Biomass Loading: Ensure your stirring mechanism has high torque capabilities to maintain consistent agitation as the mixture becomes more viscous with added lignin.
Precision in your reactor setup is the difference between a simple mixture and a chemically unified polymer.
Summary Table:
| Feature | Function in Resin Synthesis | Impact on Final Product |
|---|---|---|
| PID Heating | Maintains stable 55-65 °C window | Prevents thermal degradation & ensures high conversion |
| Continuous Stirring | Maximizes molecular collisions | Ensures uniform integration of biomass modifiers |
| Torque Control | Overcomes rising mixture viscosity | Prevents localized reaction zones & inconsistent weights |
| Alkaline Catalysis Support | Facilitates lignin/monomer bonding | Results in stable, high-quality impregnation resins |
Elevate Your Resin Synthesis with KINTEK Expertise
Achieving the perfect molecular chain for modified phenolic resins requires more than just basic equipment—it requires precision engineering. KINTEK provides industry-leading laboratory reactors designed to handle the rigorous demands of complex polymerization.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, alongside specialized lab high-temp furnaces and reactors, all customizable for your unique research needs. Whether you are focusing on biomass loading or thermal stability, our systems ensure your materials achieve superior structural integrity.
Ready to optimize your lab's efficiency? Contact us today to discuss your custom synthesis requirements!
Visual Guide
References
- Johannes Karthäuser, Holger Militz. Modification of plywood with phenol–formaldehyde resin: substitution of phenol by pyrolysis cleavage products of softwood kraft lignin. DOI: 10.1007/s00107-023-02029-z
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What is the purpose of an ultrasonic cleaner in NiMo@Cx catalyst prep? Achieve Uniform Slurry & High-Porosity Coatings
- What are alumina ceramic tubes and why are they considered advanced ceramics? Discover High-Performance Solutions for Extreme Environments
- How is quartz wool utilized in the assembly of reaction tubes? Optimize Crystal Growth and Flux Separation
- What factors should be considered when selecting a laboratory furnace? Ensure Optimal Performance and Safety
- How does a laboratory drying oven function in catalyst synthesis? Secure Precision Metal Precursor Stabilization
- Why is a high-vacuum extraction system used to reach 10⁻² Pa inside composite billets? Ensure Superior Metal Bonding
- What is the function of high-vacuum quartz sealing tubes in TiCo1-xCrxSb heat treatment? Ensure Alloy Purity
- What are the advantages of using a Boron Nitride crucible? Maximize Purity and Efficiency in Laser Pyrolysis



















