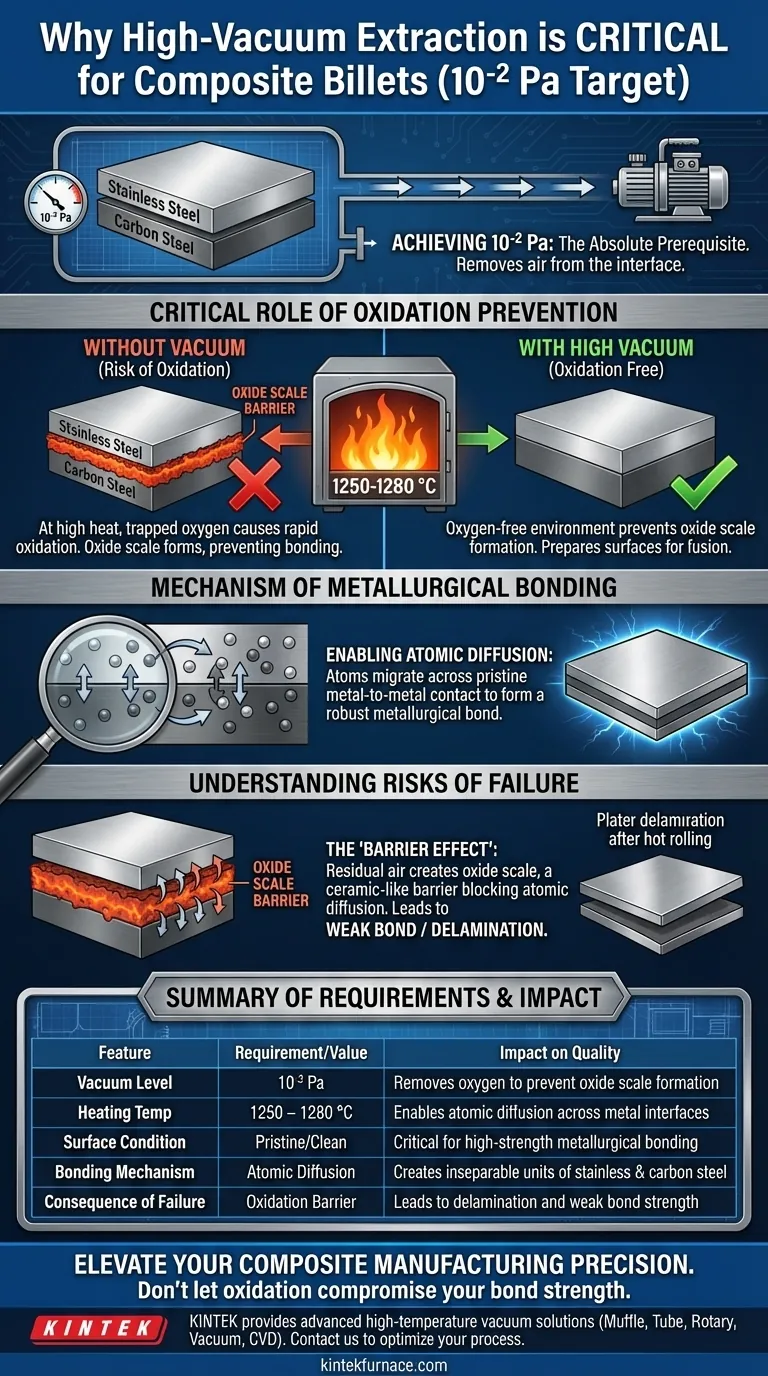
Achieving a high-vacuum state of 10⁻² Pa is the absolute prerequisite for creating a viable composite billet. This process removes air from the interface between the constituent metals (typically stainless steel and carbon steel) before they are subjected to intense heat. By creating an oxygen-free environment, you prevent the chemical reactions that would otherwise ruin the integrity of the clad plate.
The vacuum extraction system is not simply removing air; it is preparing the metal surfaces for fusion. By preventing oxide scale formation, the vacuum allows distinct metals to form a robust metallurgical bond through atomic diffusion during hot rolling.

The Critical Role of Oxidation Prevention
Eliminating the Oxygen Source
The primary objective of evacuating the billet to 10⁻² Pa is to remove oxygen from the interface.
Air trapped between the layers of steel acts as a contaminant. Even trace amounts of oxygen can trigger chemical reactions on the surface of the metals.
Surviving High Temperatures
The necessity of this vacuum becomes apparent during the heating phase.
The composite billet must be heated to temperatures between 1250 and 1280 °C. At these extreme temperatures, oxidation occurs rapidly and aggressively if oxygen is present.
Without the vacuum, the trapped air would react with the steel surfaces to form oxide scale.
The Mechanism of Metallurgical Bonding
Enabling Atomic Diffusion
The goal of the manufacturing process is to join two distinct metals into a single, inseparable unit.
This is achieved through atomic diffusion, where atoms from the stainless steel and carbon steel migrate across the interface to interlock at a microscopic level.
The Requirement for Clean Surfaces
Atomic diffusion requires pristine metal-to-metal contact.
If oxide scale forms due to a lack of vacuum, it creates a ceramic-like barrier between the layers. This barrier physically blocks atoms from diffusing, resulting in a weak or non-existent bond.
Understanding the Risks of Process Failure
The "Barrier Effect"
It is important to understand that the vacuum process does not actively bond the metals; it simply removes the obstacles to bonding.
If the system fails to reach 10⁻² Pa, residual air remains. This leads to partial oxidation, creating "dead zones" where the metals simply sit next to each other rather than fusing.
Implications for Hot Rolling
The actual bonding occurs during the subsequent hot rolling phase.
However, hot rolling cannot force oxidized surfaces to bond. If the vacuum step is skipped or insufficient, the pressure of rolling will not overcome the oxide barrier, leading to delamination (layer separation) in the final product.
Making the Right Choice for Your Goal
To ensure the structural integrity of your composite billets, focus on these operational priorities:
- If your primary focus is Bond Strength: Ensure the vacuum consistently reaches 10⁻² Pa to guarantee the atomic diffusion necessary for a robust metallurgical bond.
- If your primary focus is Process Control: Monitor the vacuum seal integrity strictly, as any leakage prior to the 1250–1280 °C heating phase will result in irreversible oxide scale formation.
A pristine, oxygen-free interface is the only foundation upon which a durable composite material can be built.
Summary Table:
| Feature | Requirement/Value | Impact on Quality |
|---|---|---|
| Vacuum Level | 10⁻² Pa | Removes oxygen to prevent oxide scale formation |
| Heating Temperature | 1250 – 1280 °C | Enables atomic diffusion across metal interfaces |
| Surface Condition | Pristine/Clean | Critical for high-strength metallurgical bonding |
| Bonding Mechanism | Atomic Diffusion | Creates inseparable units of stainless & carbon steel |
| Consequence of Failure | Oxidation Barrier | Leads to delamination and weak bond strength |
Elevate Your Composite Manufacturing Precision
Achieving the perfect metallurgical bond requires rigorous environment control. KINTEK provides the advanced high-temperature vacuum solutions needed to maintain the integrity of your materials. Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your specific lab or industrial requirements.
Don't let oxidation compromise your bond strength. Contact KINTEK today to discuss how our high-vacuum systems can optimize your composite billet production.
Visual Guide
References
- G. X. Liang, T.‐H. Chen. Interfacial Bonding Properties Experimental Research of 316L Stainless Steel–Carbon Steel Clad Rebar in the Process of Intermediate and Finish Rolling. DOI: 10.3390/met15020108
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What are the primary functions of high-purity graphite molds in SPS? Optimize Your Spark Plasma Sintering Process
- What types of high-temperature laboratory furnace systems are available? Explore 5 Specialized Solutions
- What is the significance of high-precision mass flow controllers in testing NiFe2O4? Ensure Data Integrity
- Why are high-purity alumina crucibles preferred? Secure Unmatched Purity and Data Integrity in Lab Synthesis
- What is the sucking rate for a single tap on the water circulating vacuum pump? Get Key Specs for Your Lab
- Why are high-purity alumina boats utilized as precursor containers in MoS2 synthesis? Ensure High-Quality 2D Materials
- Why are laboratory vacuum pumps and pressure gauges essential for aluminum foams? Ensure High-Quality Sintering Results
- What is the purpose of a water-cooling jacket in a methane cracking reactor? Prevent Blockages & Thermal Damage



















