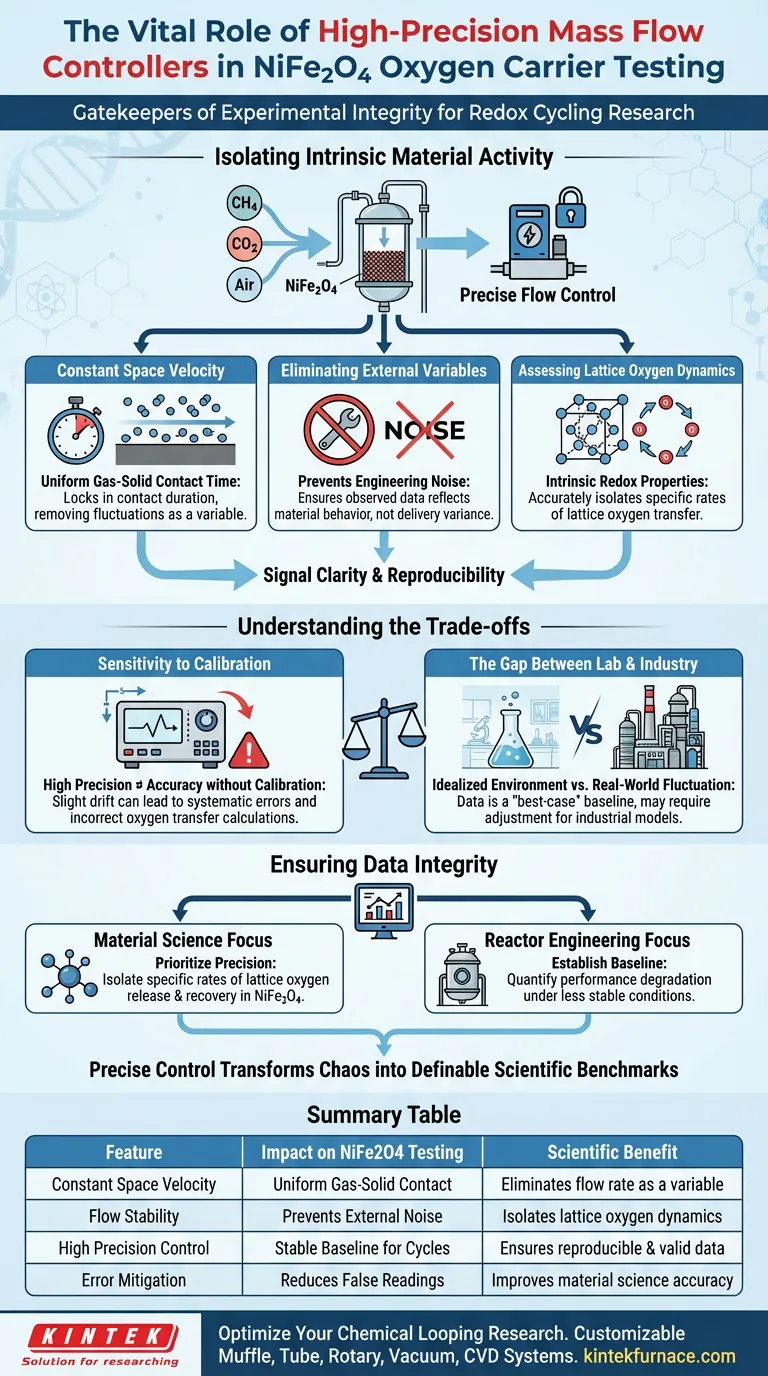
High-precision mass flow controllers are the fundamental gatekeepers of experimental integrity in chemical looping research. In the specific context of testing NiFe2O4 oxygen carriers, their primary role is to rigorously maintain a constant space velocity of reactant gases—such as methane (CH4), carbon dioxide (CO2), and air—through the reactor system. Without this strict regulation, it becomes impossible to distinguish between a change in the material’s chemical behavior and a simple fluctuation in gas delivery.
By eliminating flow rate fluctuations as a variable, these controllers isolate the experiment. This ensures that any observed data reflects the intrinsic capability of the NiFe2O4 carrier to release and recover lattice oxygen, rather than external engineering inconsistencies.

Isolating Intrinsic Material Activity
To understand the cycling performance of an oxygen carrier, you must remove the "noise" of the experimental setup. High-precision controllers are the primary tool for achieving this signal clarity.
The Critical Role of Constant Space Velocity
In redox cycling tests, the contact time between the gas and the solid oxygen carrier is a decisive factor.
If the gas flow fluctuates, the space velocity changes, altering how long reactants interact with the NiFe2O4. High-precision controllers lock this variable in place, ensuring the contact time remains uniform throughout the testing cycle.
Eliminating External Engineering Variables
Data is only valuable if it is reproducible and attributable to the material being tested.
Standard flow meters may introduce slight variances that masquerade as chemical reactivity changes. High-precision mass flow controllers eliminate these external engineering variables, preventing false positives or negatives in your performance data.
Assessing Lattice Oxygen Dynamics
The core mechanism of NiFe2O4 functioning involves the release and recovery of lattice oxygen.
Accurate assessment of these rates requires a stable baseline. By stabilizing the flow of reducing (CH4) and oxidizing (Air/CO2) gases, researchers can attribute reaction rates directly to the material's intrinsic redox properties.
Understanding the Trade-offs
While high-precision controllers are essential for accuracy, reliance on them introduces specific challenges that must be managed to maintain data integrity.
Sensitivity to Calibration
The "high precision" of these instruments makes them highly sensitive to calibration drift.
If a controller is slightly out of calibration, it will deliver a precise—but incorrect—flow rate. This can lead to systematic errors where the space velocity is constant but mathematically wrong, skewing calculations regarding oxygen transfer capacities.
The Gap Between Lab and Industry
These controllers create an idealized environment perfect for studying intrinsic material properties.
However, industrial applications rarely maintain such perfect flow stability. While necessary for determining intrinsic material limits, data derived under these pristine conditions may need adjustment when modeling for large-scale, fluctuating industrial reactors.
Ensuring Data Integrity in Redox Cycles
When designing your experimental apparatus or analyzing cycling data, the quality of flow control dictates the validity of your conclusions regarding the oxygen carrier.
- If your primary focus is Fundamental Material Science: Prioritize flow precision to isolate the specific rates of lattice oxygen release and recovery within the NiFe2O4 crystal structure.
- If your primary focus is Reactor Engineering: Use high-precision control to establish a "best-case" baseline, allowing you to later quantify how much performance degrades under less stable industrial conditions.
Ultimately, precise flow control is what transforms a chaotic chemical reaction into a measurable, definable scientific benchmark.
Summary Table:
| Feature | Impact on NiFe2O4 Testing | Scientific Benefit |
|---|---|---|
| Constant Space Velocity | Maintains uniform gas-solid contact time | Eliminates flow rate as a variable |
| Flow Stability | Prevents external engineering noise | Isolates lattice oxygen dynamics |
| High Precision Control | Delivers stable baseline for redox cycles | Ensures reproducible & valid data |
| Error Mitigation | Reduces false performance readings | Improves material science accuracy |
Optimize Your Chemical Looping Research with KINTEK
Precise experimental data begins with high-performance laboratory equipment. KINTEK provides the advanced thermal and gas-handling solutions necessary to isolate intrinsic material properties and achieve reproducible results.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, CVD systems, and other laboratory high-temperature furnaces—all fully customizable to meet the rigorous demands of oxygen carrier testing and redox cycling research.
Ready to elevate your lab's precision? Contact our experts today to discuss how our customizable systems can support your unique research goals.
Visual Guide
References
- Da Song, Fanxing Li. Unraveling the atomic interdiffusion mechanism of NiFe<sub>2</sub>O<sub>4</sub> oxygen carriers during chemical looping CO<sub>2</sub> conversion. DOI: 10.1002/cey2.493
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 304 316 Stainless Steel High Vacuum Ball Stop Valve for Vacuum Systems
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
- Ultra Vacuum Electrode Feedthrough Connector Flange Power Lead for High Precision Applications
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Cylindrical Resonator MPCVD Machine System for Lab Diamond Growth
People Also Ask
- Why is it necessary to achieve a vacuum level of 3 x 10^-2 mm Hg for quartz tube sealing? Ensure Safety and Purity
- What role does a heated substrate platform play in the spray pyrolysis deposition? Optimize Your Thin Film Quality
- How are laboratory vacuum pumps utilized in 1T-TaS2 crystal preparation? Ensure Peak Sample Purity
- How does an evaporation and mixing unit assist in fuel ignition study? Enhance Research with Precise Vapor Control
- Why is a laboratory-grade high-pressure reactor essential for TiO2 nanoparticles? Optimize Purity and Efficiency
- What are the roles of laboratory vacuum drying ovens and precision analytical balances in moisture monitoring?
- How is the vacuuming operation performed with a water circulating vacuum pump? Master the Liquid Ring Technique
- What role does a high-purity alumina crucible play in BSO synthesis? Ensure Purity in High-Temperature Reactions



















