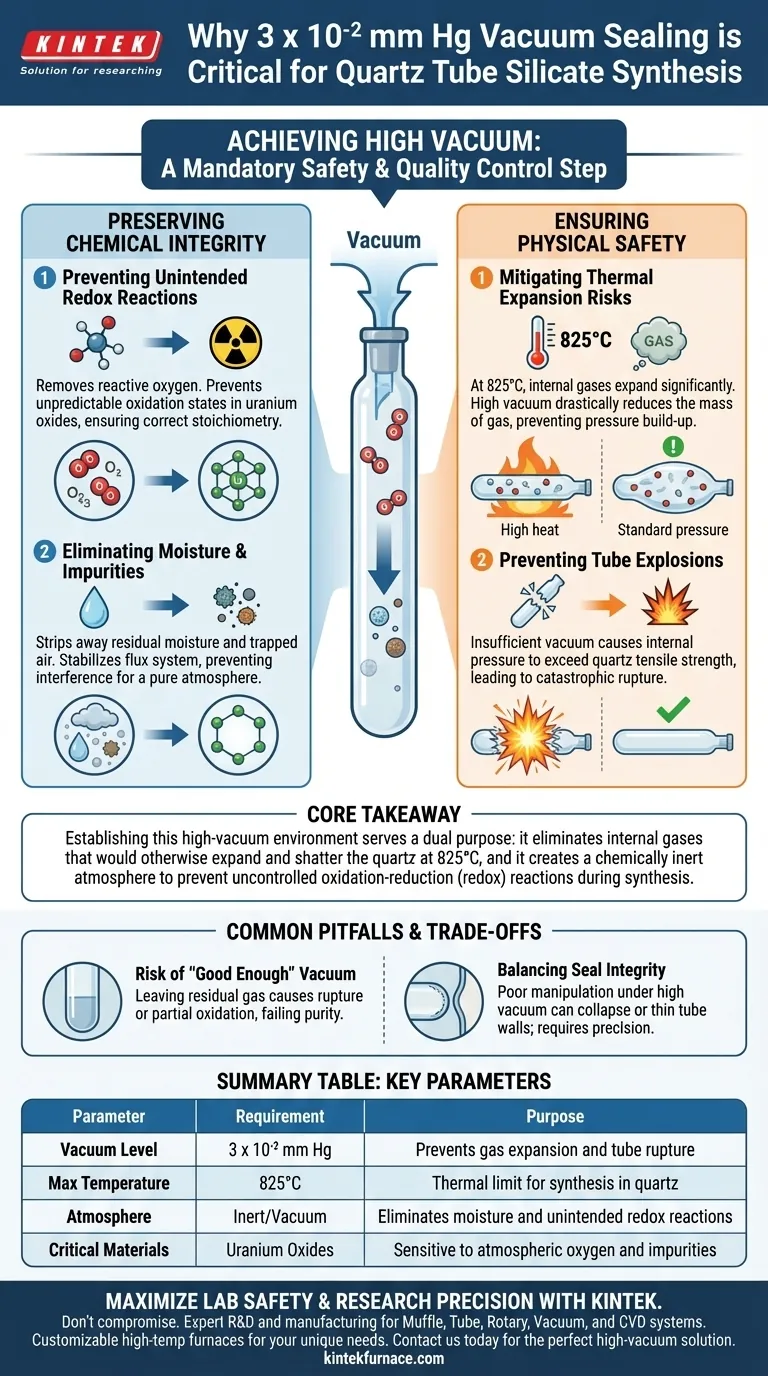
Achieving a vacuum level of 3 x 10⁻² mm Hg is a mandatory safety and quality control step. This specific pressure threshold is required to effectively evacuate air and moisture from the quartz tube, preventing catastrophic structural failure during heating and ensuring the chemical stability of sensitive compounds like uranium oxides.
Core Takeaway Establishing this high-vacuum environment serves a dual purpose: it eliminates internal gases that would otherwise expand and shatter the quartz at 825°C, and it creates a chemically inert atmosphere to prevent uncontrolled oxidation-reduction (redox) reactions during synthesis.

Preserving Chemical Integrity
Preventing Unintended Redox Reactions
In the context of silicate synthesis involving uranium oxides, the presence of atmospheric oxygen is detrimental. Achieving a vacuum of 3 x 10⁻² mm Hg removes the reactive oxygen that would otherwise trigger unintended redox reactions.
Without this vacuum, the oxidation state of the uranium could shift unpredictably, altering the final stoichiometry and properties of the synthesized silicate.
Eliminating Moisture and Impurities
The vacuum process is essential for stripping away residual moisture and air trapped within the tube.
If moisture remains, it can destabilize the flux system, preventing it from operating in a pure atmosphere. A dry, evacuated environment ensures that the interaction between the reactants and the flux proceeds exactly as chemically intended, without interference from water vapor.
Ensuring Physical Safety
Mitigating Thermal Expansion Risks
The synthesis process involves heating the quartz tube to temperatures as high as 825°C.
According to gas laws, any gas remaining inside a sealed vessel will expand significantly when heated. By reducing the internal pressure to 3 x 10⁻² mm Hg before sealing, you drastically reduce the mass of gas present.
Preventing Tube Explosions
The most immediate physical danger of insufficient vacuum is quartz tube explosion.
If the tube contains standard atmospheric pressure (or insufficient vacuum) when sealed, the internal pressure generated at 825°C will exceed the tensile strength of the quartz. The high vacuum creates a safety buffer, ensuring the internal pressure remains low enough to maintain the structural integrity of the vessel throughout the heating cycle.
Common Pitfalls and Trade-offs
The Risk of "Good Enough" Vacuum
A common error is stopping the evacuation process before reaching the 3 x 10⁻² mm Hg threshold.
While a lower-quality vacuum might seem sufficient to seal the glass, it often leaves behind enough residual gas to cause a rupture at peak temperatures. Furthermore, trace amounts of remaining oxygen can lead to partial oxidation, resulting in a heterogeneous product that fails purity standards.
Balancing Seal Integrity
While high vacuum is critical, the sealing process itself must be precise.
If the quartz is manipulated poorly while under high vacuum, the walls can collapse inward or thin out excessively. The technician must ensure the seal is robust enough to hold the vacuum without compromising the tube's thickness at the seal point.
Making the Right Choice for Your Goal
To ensure the success of your silicate synthesis, align your vacuum procedures with your specific objectives:
- If your primary focus is Personnel Safety: Prioritize the vacuum level to prevent gas expansion; any pressure above 3 x 10⁻² mm Hg increases the risk of the quartz tube exploding at 825°C.
- If your primary focus is Chemical Purity: Ensure the vacuum is stable to remove all moisture and oxygen, which is the only way to prevent unintended redox reactions in uranium oxides.
Ultimately, this vacuum level is not an arbitrary variable; it is the fundamental barrier between a successful reaction and a hazardous failure.
Summary Table:
| Parameter | Requirement | Purpose |
|---|---|---|
| Vacuum Level | 3 x 10⁻² mm Hg | Prevents gas expansion and tube rupture |
| Max Temperature | 825°C | Thermal limit for synthesis in quartz |
| Atmosphere | Inert/Vacuum | Eliminates moisture and unintended redox reactions |
| Critical Materials | Uranium Oxides | Sensitive to atmospheric oxygen and impurities |
Maximize Lab Safety and Research Precision with KINTEK
Don't compromise your silicate synthesis with sub-par vacuum environments. At KINTEK, we understand that precision is the barrier between a successful reaction and a hazardous failure. Backed by expert R&D and manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable to meet your unique high-temperature laboratory needs.
Whether you are synthesizing sensitive uranium oxides or conducting advanced material research, our high-temp furnaces provide the stability and control you require.
Ready to elevate your lab's capabilities? Contact us today to consult with our experts on the perfect high-vacuum solution for your application.
Visual Guide
References
- Еvgeny V. Nazarchuk, Dmitri O. Charkin. A novel microporous uranyl silicate prepared by high temperature flux technique. DOI: 10.1515/zkri-2024-0121
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Ultra High Vacuum Stainless Steel KF ISO CF Flange Pipe Straight Pipe Tee Cross Fitting
- 304 316 Stainless Steel High Vacuum Ball Stop Valve for Vacuum Systems
- Stainless Steel Quick Release Vacuum Chain Three Section Clamp
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- Why are support frames important for the alumina furnace tube? Prevent High-Temperature Deformation and Failure
- Why is a benchtop forced air drying oven preferred for microalgae-based nanomaterials? Enhance Powder Quality
- Why are alumina or ceramic crucibles selected for KCdCl3 perovskite? Ensure High Purity and Thermal Stability
- How does the selection of high-temperature crucibles impact the Sintering Dissolution Process (SDP)?
- What is the critical role of the vacuum filter in a waste magnesium vacuum distillation system? The Essential Protection for Your Vacuum Pump
- What are the primary applications of alumina ceramic tubes? Ideal for High-Temp, Corrosive, and Insulating Needs
- What is the specific function of the water circulation cooler in zirconium sponge processing? Key for Purity & Safety
- What is the role of quartz capillaries in the vacuum sealing process of sulfur? Enhance Purity and In-Situ Analysis



















