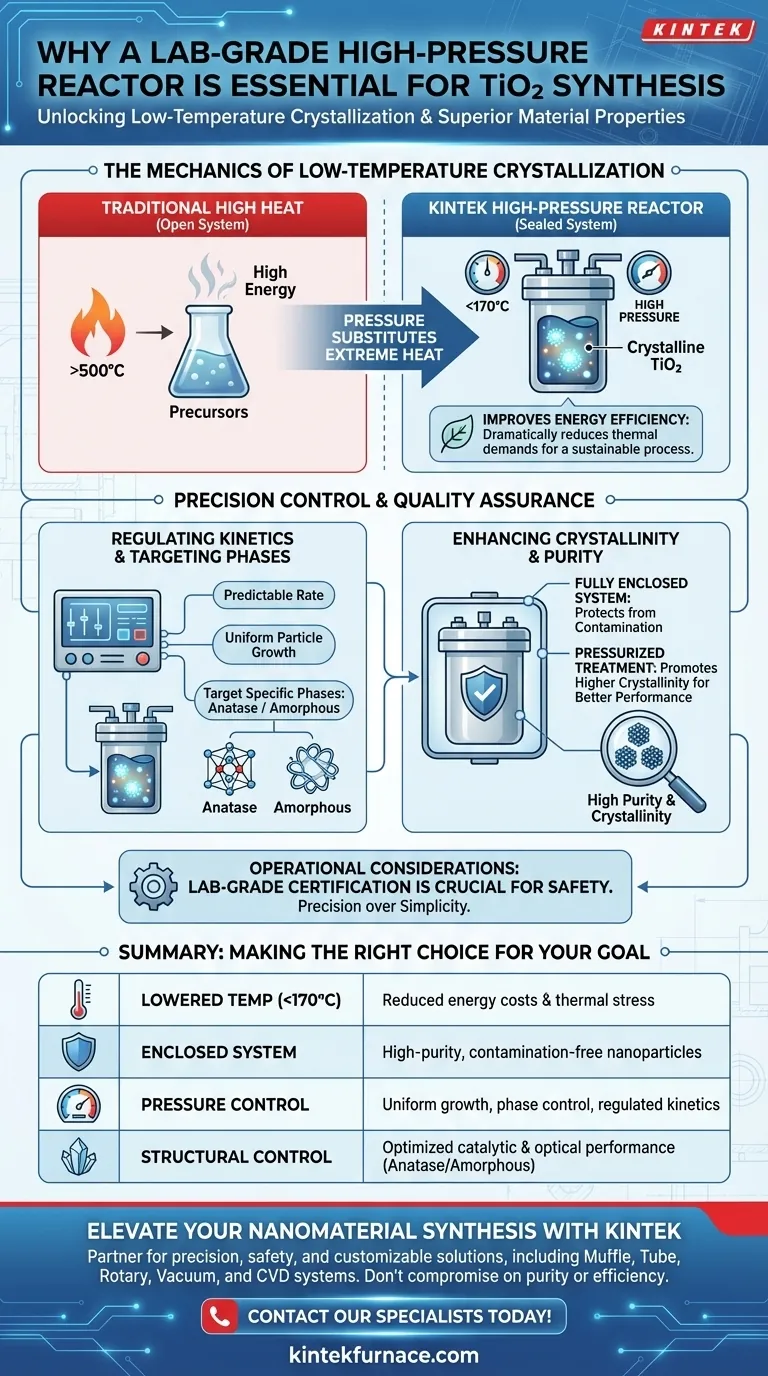
A laboratory-grade high-pressure reactor is the indispensable tool for accessing the specific thermodynamic conditions required to crystallize titanium precursors effectively. By providing a controlled pressure environment, this equipment enables synthesis at temperatures significantly lower than traditional methods—often below 170°C. This capability is essential for managing reaction kinetics to produce high-purity, crystalline TiO2 nanoparticles without the excessive energy demands of solid-state processing.
By leveraging a sealed, pressurized environment, these reactors decouple crystallization from high heat, allowing for the precise synthesis of specific TiO2 phases with superior purity and energy efficiency.

The Mechanics of Low-Temperature Crystallization
Utilizing Pressure to Lower Thermal Demands
The primary function of the high-pressure reactor is to substitute extreme heat with pressure.
In an open system, crystallization often requires intense thermal energy. However, within a sealed reactor, the elevated pressure allows titanium precursors to crystallize at temperatures below 170°C.
Improving Energy Efficiency
This dramatic reduction in processing temperature represents a significant shift from traditional solid-state synthesis.
By operating effectively at lower temperatures, the reactor minimizes the overall energy consumption of the process. This makes the hydrothermal or rotating autoclave method far more sustainable than high-heat alternatives.
Precision Control Over Material Properties
Regulating Reaction Kinetics
Achieving a high-quality nanomaterial requires strict command over how fast the reaction proceeds.
The enclosed system of a laboratory-grade reactor provides precise control over reaction kinetics. This ensures the chemical transformation occurs at a predictable rate, which is vital for uniform particle growth.
Targeting Specific Crystalline Phases
Titanium dioxide (TiO2) can exist in different structural forms, which determine its utility.
The controlled environment allows you to steer the synthesis toward specific desired phases, such as amorphous or anatase TiO2. Without the containment and pressure of this specific reactor, isolating these specific phases becomes incredibly difficult.
Purity and Quality Assurance
Enhancing Crystallinity
The ultimate goal of using a high-pressure reactor is to improve the internal structure of the nanoparticle.
The pressurized treatment directly promotes higher crystallinity in the final product. A highly crystalline structure is often essential for the catalytic or optical performance of TiO2.
Ensuring Product Purity
Contamination is a major risk in open-air synthesis methods.
Because the reactor operates as a fully enclosed system, it protects the reaction from external contaminants. This isolation guarantees a higher purity level in the final TiO2 nanoparticles.
Operational Considerations and Trade-offs
The Necessity of Laboratory-Grade Equipment
While the benefits are clear, it is crucial to recognize that standard vessels cannot substitute for this equipment.
Attempting to replicate these conditions in non-rated vessels poses severe safety risks due to the pressure involved. Laboratory-grade certification ensures the vessel can safely sustain the internal pressures required to drive the kinetics at low temperatures.
Complexity vs. Simplicity
Using a high-pressure reactor adds a layer of operational complexity compared to simple precipitation methods.
However, this complexity is the "cost" of accessing superior material properties. If you prioritize simple, ambient-pressure equipment, you sacrifice the ability to achieve high crystallinity at low temperatures.
Making the Right Choice for Your Goal
To maximize the effectiveness of your TiO2 synthesis, align your equipment choice with your specific research or production targets:
- If your primary focus is Energy Efficiency: Utilize the reactor to exploit the ability to crystallize precursors at temperatures below 170°C, significantly cutting thermal costs.
- If your primary focus is Phase Purity: Leverage the controlled pressure environment to target specific phases like anatase, ensuring the material meets precise structural specifications.
Ultimately, the high-pressure reactor is not just a vessel, but a precision instrument that grants you control over the fundamental physics of TiO2 crystallization.
Summary Table:
| Feature | Benefit for TiO2 Synthesis | Impact on Final Product |
|---|---|---|
| Lowered Temperature | Crystallization below 170°C | Reduced energy costs and thermal stress |
| Enclosed System | Contamination-free environment | High-purity crystalline nanoparticles |
| Pressure Control | Regulation of reaction kinetics | Uniform particle growth and phase control |
| Structural Control | Targeting Anatase or Amorphous phases | Optimized catalytic and optical performance |
Elevate Your Nanomaterial Synthesis with KINTEK
Precision is non-negotiable when crystallizing TiO2 phases. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, alongside specialized laboratory-grade high-pressure reactors—all fully customizable to meet your unique research needs.
Don't compromise on safety or material purity. Partner with KINTEK to access the thermal and pressure control necessary for superior crystallinity and energy efficiency.
Contact our specialists today to find the perfect solution for your lab!
Visual Guide
References
- A. C. W. W. M. N. Peshala Koswatta, Atula S. D. Sandanayaka. Boosting Solar Cell Efficiency: Enhancing Dye-Sensitized Solar Cell Performance with Carbon Quantum Dots and Titanium Dioxide Nanostructures from Sri Lankan Ilmenite. DOI: 10.1021/acsomega.5c02272
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant Rotating Furnace
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- What are the functions of high-purity, high-strength graphite molds in SPS? Optimize Al2O3-TiC Ceramic Sintering
- What is the function of high-purity graphite crucibles in Al-Cu-Mn master alloy prep? Ensure Chemical Purity
- What is the purpose of using integrated temperature controllers for CuInP2S6? Master CIPS Electrical Characterization
- What key functions do graphite molds serve during the hot press sintering? Enhance Ti/Al2O3 Composite Quality
- How does an infrared pyrometer facilitate the precise control of temperatures during microwave-assisted metal recovery?
- How does the hardness of alumina ceramics compare to other materials? Discover Its Superior Wear Resistance
- Is a work tube included with the furnace? Customize Your Setup for Optimal Performance
- What is the function of an in-situ heating holder in the study of Peierls transitions in NaRu2O4? Dynamic Lab Insights



















