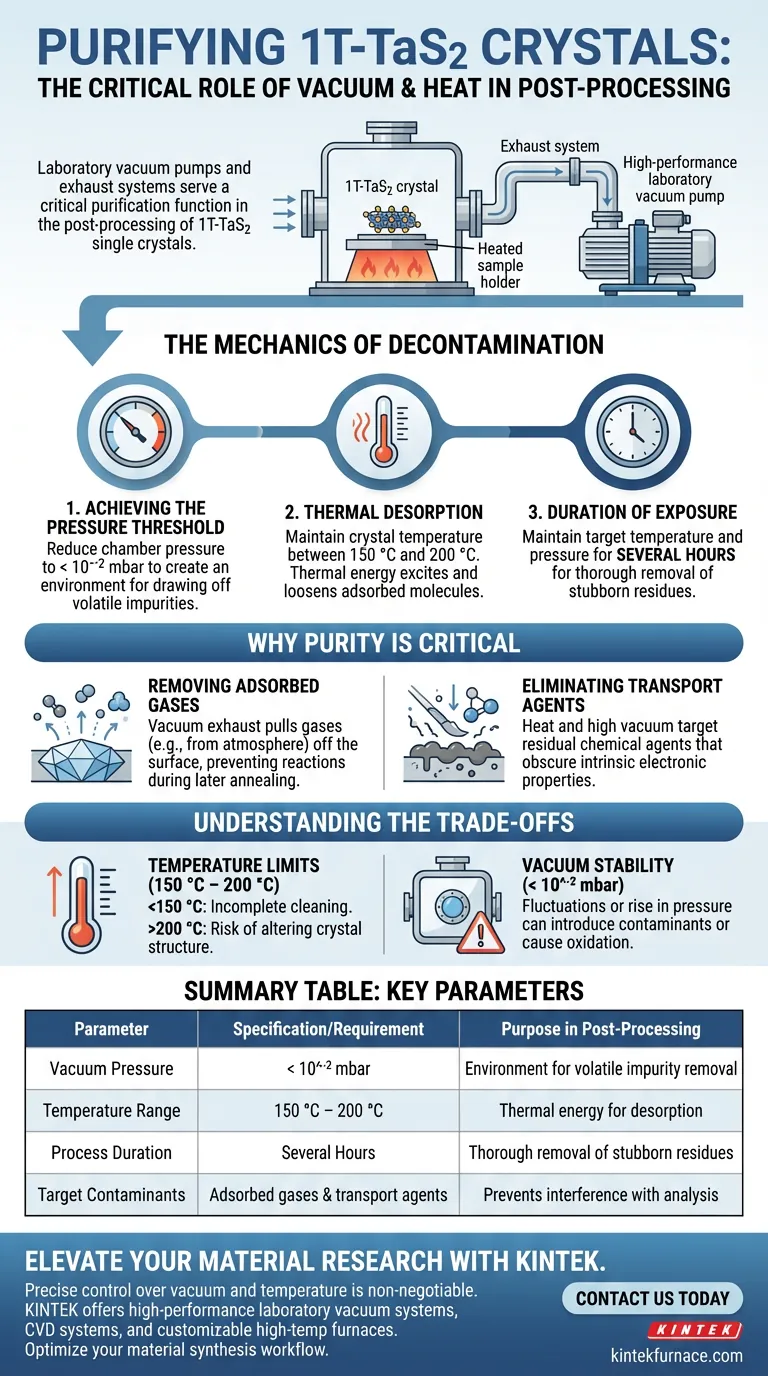
Laboratory vacuum pumps and exhaust systems serve a critical purification function in the post-processing of 1T-TaS2 single crystals. These systems are utilized to transfer crystals into a controlled environment with pressures lower than 10^-2 mbar. By maintaining this high vacuum while simultaneously heating the samples to between 150 °C and 200 °C for several hours, the equipment effectively strips away surface contaminants prior to the annealing phase.
The primary role of these vacuum systems is to eliminate environmental impurities and residual transport agents, ensuring that subsequent electronic structure characterizations reflect the true properties of the material rather than surface contamination.
The Mechanics of Decontamination
Achieving the Pressure Threshold
To effectively prepare 1T-TaS2 crystals, the vacuum system must reduce the chamber pressure to lower than 10^-2 mbar.
This specific pressure threshold is required to create an environment where volatile impurities can be drawn off the surface of the crystal. Without reaching this low pressure, surface contaminants remain stable and interfere with the sample's purity.
Thermal Desorption
Vacuum pumping alone is often insufficient for deep cleaning; it must be paired with thermal energy.
The process involves maintaining the crystals at a temperature between 150 °C and 200 °C. This thermal input excites adsorbed molecules, loosening their bond with the crystal surface so the vacuum pump can remove them.
Duration of Exposure
This is not a rapid process. The system must maintain the target temperature and pressure for several hours.
This sustained duration ensures that the desorption process is thorough, removing stubborn residues rather than just the most volatile surface layers.
Why Purity Is Critical
Removing Adsorbed Gases
1T-TaS2 crystals are sensitive to their environment. During handling, they naturally adsorb gases from the atmosphere.
The vacuum exhaust system acts as a stripping mechanism, pulling these gases off the crystal surface to preventing them from reacting with the material during later high-temperature annealing steps.
Eliminating Transport Agents
Crystal synthesis often leaves behind residual transport agents.
These chemical residues can obscure the intrinsic electronic properties of the crystal. The combination of heat and high vacuum specifically targets these residues, ensuring the crystal surface is chemically clean for analysis.
Understanding the Trade-offs
Temperature Limits
While heat aids in cleaning, precise control is essential.
The process operates strictly between 150 °C and 200 °C. Deviating below this range may result in incomplete cleaning, while exceeding it could risk altering the crystal structure before the controlled annealing phase begins.
Vacuum Stability
The effectiveness of this process relies entirely on the stability of the vacuum seal.
If the pressure fluctuates or rises above 10^-2 mbar during the heating phase, the system may inadvertently introduce new contaminants or cause oxidation, rendering the hours of preparation wasted.
Making the Right Choice for Your Goal
To ensure your 1T-TaS2 samples are correctly prepared for analysis, align your vacuum workflow with your specific objectives:
- If your primary focus is maximizing surface purity: Maintain the temperature closer to the 200 °C upper limit for the full duration to ensure the complete volatilization of stubborn transport agents.
- If your primary focus is baseline data integrity: prioritize verifying that the pressure remains consistently below 10^-2 mbar throughout the thermal cycle to prevent environmental re-contamination.
Ultimately, the vacuum system acts as the gatekeeper of data quality, transforming a raw synthesized crystal into a reliable sample ready for precise electronic analysis.
Summary Table:
| Parameter | Specification/Requirement | Purpose in Post-Processing |
|---|---|---|
| Vacuum Pressure | < 10^-2 mbar | Creates an environment for volatile impurity removal |
| Temperature Range | 150 °C – 200 °C | Provides thermal energy for desorption of molecules |
| Process Duration | Several Hours | Ensures thorough removal of stubborn surface residues |
| Target Contaminants | Adsorbed gases & transport agents | Prevents interference with electronic property analysis |
Elevate Your Material Research with KINTEK
Precise control over vacuum and temperature is non-negotiable for high-performance crystal preparation. Backed by expert R&D and manufacturing, KINTEK offers high-performance laboratory vacuum systems, CVD systems, and customizable high-temp furnaces designed to meet the rigorous demands of 1T-TaS2 processing and beyond.
Don't let surface impurities compromise your data integrity. Contact us today to find the perfect customizable solution for your lab and see how our advanced heating and vacuum technology can optimize your material synthesis workflow.
Visual Guide
References
- Yihao Wang, Liang Cao. Dualistic insulator states in 1T-TaS2 crystals. DOI: 10.1038/s41467-024-47728-0
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What is the core function of a planetary ball mill in Bi2Te3 alloying? Drive Solid-State Reaction & Nanoscale Refinement
- Why is a laboratory vacuum degasser necessary for biochar? Ensure Accurate BET Structural Characterization
- What is the function of a ceramic crucible with a lid during g-C3N4 synthesis? Optimize Your Polycondensation Results
- How does surface finish impact the performance of alumina ceramic furnace tubes? Boost Purity and Efficiency
- What role does a rotary evaporator serve in the processing of banana inflorescence extracts? Maximize Bioactive Recovery
- What is the temperature resistance of alumina ceramic tubes? Up to 1800°C for Demanding Applications
- What role does a laboratory vacuum pump play in a static batch desulfurization evaluation system? Ensure Data Integrity
- What are the material requirements for the core reaction chambers? Ensure Pure Pyrolysis with Quartz and Corundum



















