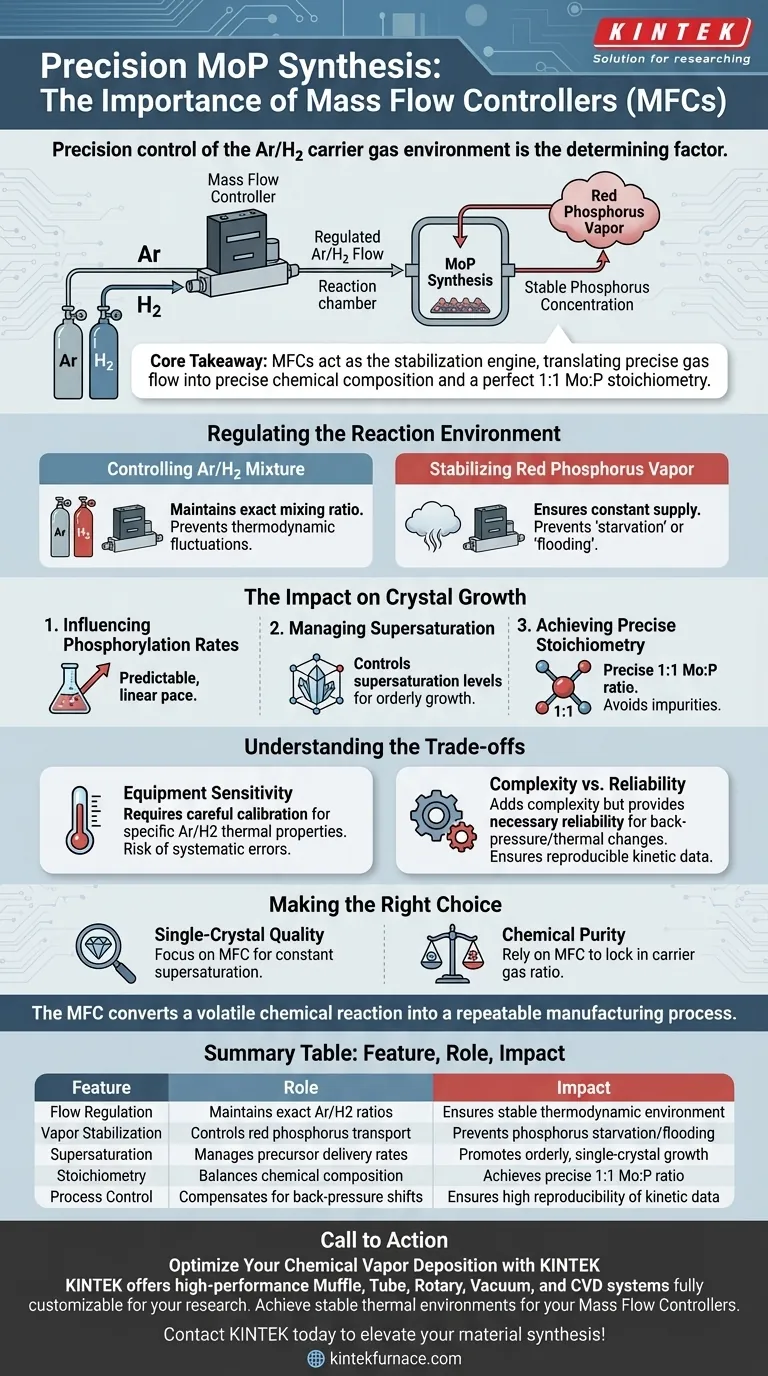
Precision control of the carrier gas environment is the determining factor in synthesizing high-quality Molybdenum Phosphide (MoP).
The primary importance of using a Mass Flow Controller (MFC) in this process is to strictly regulate the flow rate and ratio of the Ar/H2 gas mixture. By maintaining a stable carrier gas flow, the MFC ensures a constant concentration of red phosphorus vapor within the reaction chamber, which is the fundamental requirement for controlled crystal growth.
Core Takeaway The Mass Flow Controller acts as the stabilization engine for the entire synthesis, translating precise gas flow into precise chemical composition. It eliminates environmental fluctuations to ensure the final material achieves a perfect 1:1 Mo:P stoichiometry and consistent physical structure.

Regulating the Reaction Environment
Controlling the Ar/H2 Mixture
The synthesis of MoP relies on a carrier gas, typically a mixture of Argon (Ar) and Hydrogen (H2), to transport precursors.
An MFC is essential because it maintains the exact mixing ratio of these gases throughout the duration of the experiment. Even minor fluctuations in the gas ratio can alter the thermodynamic environment, disrupting the reaction.
Stabilizing Red Phosphorus Vapor
The Ar/H2 mixture acts as a vehicle for transporting red phosphorus vapor to the reaction site.
If the carrier gas flow varies, the concentration of phosphorus vapor reaching the substrate becomes inconsistent. The MFC ensures a constant supply of phosphorus, preventing "starvation" or "flooding" of the reaction zone.
The Impact on Crystal Growth
Influencing Phosphorylation Rates
The rate at which molybdenum is converted into molybdenum phosphide is defined as the phosphorylation rate.
This rate is directly dictated by the stability of the gas environment provided by the MFC. A steady flow ensures the chemical reaction proceeds at a predictable, linear pace rather than in uncontrolled bursts.
Managing Supersaturation
Crystal growth requires a specific state known as supersaturation, where the vapor pressure exceeds the equilibrium limit.
The MFC controls the supersaturation levels by regulating how much precursor is delivered at any given moment. This control allows the crystal to grow in an orderly fashion, rather than precipitating randomly.
Achieving Precise Stoichiometry
The ultimate goal of MoP synthesis is often to create single-crystal samples with a specific chemical makeup.
The MFC is vital for producing samples with a precise Mo:P ratio of approximately 1:1. Without the strict regulation of the carrier gas, the stoichiometry often drifts, resulting in impurities or chemically uneven samples.
Understanding the Trade-offs
Equipment Sensitivity
While MFCs offer superior precision, they are sensitive to calibration and contamination.
If the MFC is not calibrated for the specific thermal properties of the Ar/H2 mixture, the flow readings may be inaccurate. This can lead to systematic errors in the resulting stoichiometry, even if the readout appears stable.
Complexity vs. Reliability
Using an MFC adds complexity to the setup compared to simple rotameters, but it provides necessary reliability.
Manual flow control cannot react to back-pressure changes or thermal shifts in the way an MFC can. Skipping the MFC compromises the reproducibility of kinetic data, making it impossible to distinguish between experimental error and actual physical phenomena.
Making the Right Choice for Your Goal
To maximize the quality of your MoP synthesis, align your equipment usage with your specific objectives:
- If your primary focus is Single-Crystal Quality: Prioritize the MFC's ability to maintain constant supersaturation, as this dictates the morphological consistency of the final sample.
- If your primary focus is Chemical Purity: Rely on the MFC to lock in the carrier gas ratio, ensuring the 1:1 stoichiometry is not compromised by fluctuating phosphorus vapor concentrations.
The MFC is not just a flow regulator; it is the critical tool that converts a volatile chemical reaction into a repeatable manufacturing process.
Summary Table:
| Feature | Role in MoP Synthesis | Impact on Material Quality |
|---|---|---|
| Flow Regulation | Maintains exact Ar/H2 mixing ratios | Ensures stable thermodynamic environment |
| Vapor Stabilization | Controls red phosphorus transport | Prevents phosphorus starvation or flooding |
| Supersaturation | Manages precursor delivery rates | Promotes orderly, single-crystal growth |
| Stoichiometry | Balances chemical composition | Achieves precise 1:1 Mo:P atomic ratio |
| Process Control | Compensates for back-pressure shifts | Ensures high reproducibility of kinetic data |
Optimize Your Chemical Vapor Deposition with KINTEK
Precision in MoP synthesis starts with reliable high-temperature systems and gas control. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique research or production needs.
Whether you are aiming for perfect single-crystal quality or 1:1 chemical stoichiometry, our advanced lab furnaces provide the stable thermal environment your Mass Flow Controllers need to succeed. Contact KINTEK today to discuss your custom furnace requirements and elevate your material synthesis to the next level!
Visual Guide
References
- Seo Hyun Kim, Hyeuk Jin Han. Facet‐Controlled Growth of Molybdenum Phosphide Single Crystals for Efficient Hydrogen Peroxide Synthesis. DOI: 10.1002/adma.202500250
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
- Spark Plasma Sintering SPS Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
- HFCVD Machine System Equipment for Drawing Die Nano Diamond Coating
People Also Ask
- Why are alumina ceramic tubes preferred for high-temperature furnaces? Ensure Stability and Control Up to 1800°C
- How does the density of alumina ceramics compare to steel? Uncover Lighter, High-Performance Material Solutions
- What are the key mechanical properties of alumina tubes? Uncover High-Strength, Wear-Resistant Solutions
- Why use R-type and K-type thermocouples for slag measurement? Optimize High-Temp Thermal Profiling and Modeling
- What is the impact of gas flow meters on catalyst synthesis? Ensure Phase Purity and Precision in (NiZnMg)MoN Production
- Why must a high-purity quartz boat be used for Co-Fe-NC catalyst pyrolysis? Ensure Peak Catalyst Purity
- Why is an additional large alumina outer crucible required? Ensure Safety and Equipment Longevity in Steel Research
- Why is a graphite thermal baffle necessary for thermal field control? Master Single-Crystal Growth Quality



















