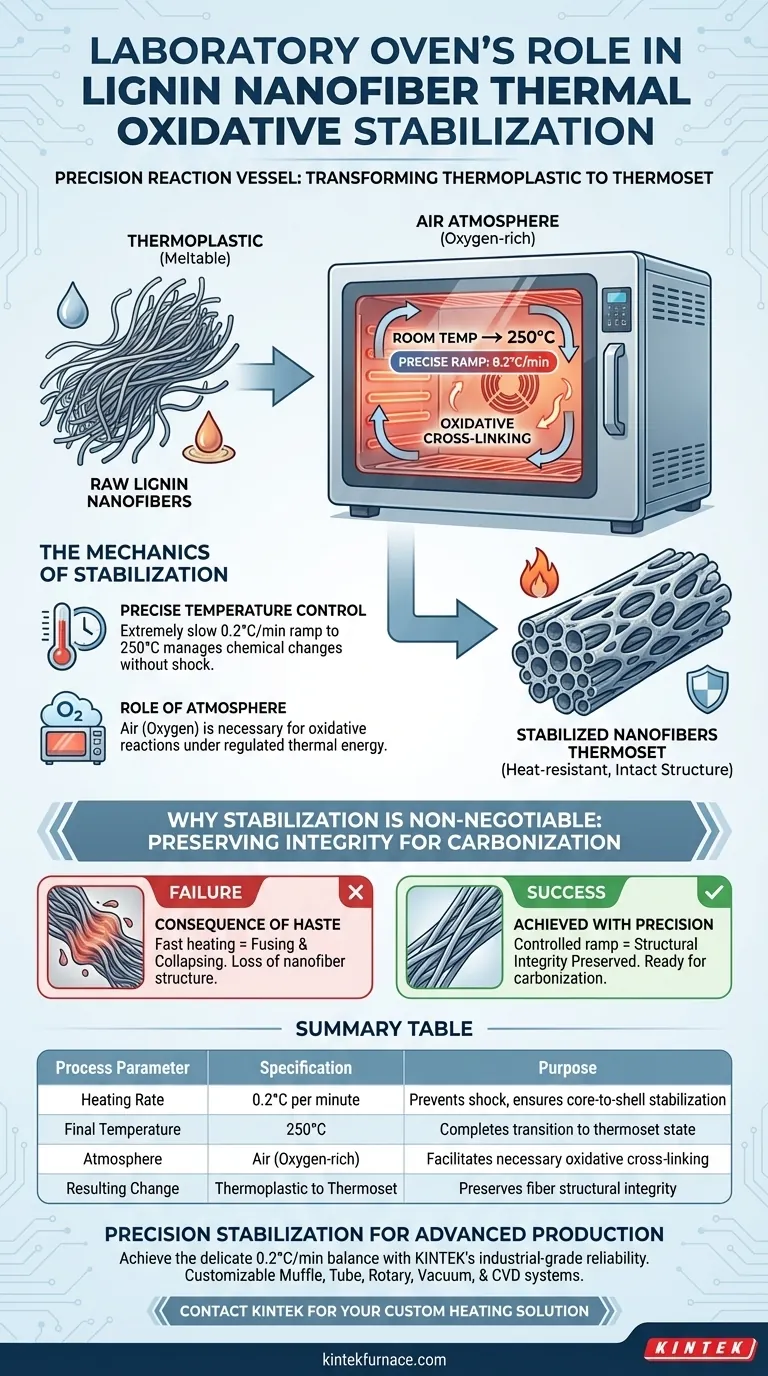
The laboratory oven acts as a precision reaction vessel regarding the thermal oxidative stabilization of lignin nanofiber membranes. Its role is to execute a strictly controlled heating program—specifically ramping from room temperature to 250 degrees Celsius at a slow rate of 0.2 degrees Celsius per minute—within an air atmosphere. This controlled environment drives the chemical cross-linking required to convert the fibers from a meltable state into a heat-resistant structure.
The laboratory oven’s primary function is to transform the lignin nanofibers from a thermoplastic state to a thermoset state. This stabilization phase prevents the fibers from fusing or collapsing during carbonization, preserving the membrane's structural integrity.

The Mechanics of Stabilization
Precise Temperature Control
The stabilization process relies on an extremely slow and steady temperature increase. The laboratory oven is programmed to raise the temperature at a rate of just 0.2 degrees Celsius per minute.
This slow ramp continues until the chamber reaches 250 degrees Celsius. This gradual heating is essential to manage the chemical changes occurring within the fiber without shocking the material.
The Role of Atmosphere
Unlike vacuum or inert gas ovens, this process utilizes an air atmosphere.
The presence of oxygen is necessary to facilitate the oxidative reactions. The oven maintains this environment while strictly regulating the thermal energy applied to the nanofibers.
Why Stabilization is Non-Negotiable
From Thermoplastic to Thermoset
Raw lignin nanofibers are naturally thermoplastic. This means that if exposed to high heat without preparation, they will soften and flow like a liquid.
The oven facilitates cross-linking between the fiber molecules. This chemical change converts the material into a thermoset structure, which hardens under heat rather than melting.
Preserving Structural Integrity
The ultimate goal of using the oven is to prepare the material for subsequent high-temperature carbonization.
If the fibers melt or collapse during stabilization, the distinct nanofiber structure is lost. The oven ensures the fibers maintain their shape and integrity, preventing them from fusing into a solid mass during later processing steps.
Critical Process Variables and Risks
The Consequence of Haste
The specific ramp rate (0.2°C/min) is not a suggestion; it is a critical parameter.
If the oven heats the material too quickly, the outer shell of the fiber may stabilize while the core remains thermoplastic. This can lead to defects or structural failure when the temperature rises further.
Temperature Uniformity
The oven must maintain uniform heat distribution throughout the chamber. Cold spots or fluctuations can result in uneven cross-linking, leaving parts of the membrane vulnerable to melting.
Making the Right Choice for Your Goal
To ensure the production of high-quality lignin nanofiber membranes, consider the following regarding your stabilization equipment:
- If your primary focus is Structural Integrity: Ensure your oven can maintain the strict 0.2°C/min ramp rate without fluctuation to guarantee complete thermoplastic-to-thermoset conversion.
- If your primary focus is Process Consistency: Verify that airflow within the oven is sufficient to supply the necessary oxygen for oxidative cross-linking across the entire membrane surface.
Ultimately, the laboratory oven provides the precise thermal environment required to lock in the nanofiber structure, making the final carbonization phase possible.
Summary Table:
| Process Parameter | Specification | Purpose |
|---|---|---|
| Heating Rate | 0.2°C per minute | Prevents material shock and ensures core-to-shell stabilization |
| Final Temperature | 250°C | Completes the transition to a heat-resistant thermoset state |
| Atmosphere | Air (Oxygen-rich) | Facilitates necessary oxidative chemical cross-linking |
| Resulting Change | Thermoplastic to Thermoset | Preserves fiber structural integrity during carbonization |
Precision Stabilization for Advanced Nanofiber Production
Achieving the delicate balance of 0.2°C/min ramp rates requires industrial-grade reliability and thermal uniformity. KINTEK provides high-performance laboratory solutions tailored for complex materials research. Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your specific stabilization and carbonization needs.
Don't compromise the structural integrity of your lignin membranes. Contact KINTEK today to find your custom heating solution.
Visual Guide
References
- Reima Herrala, Jaana Vapaavuori. Functionalizing Lignin‐Based Nanofiber Electrodes with Gold Using Electrochemically Assisted Aqueous Reduction. DOI: 10.1002/admi.202400748
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- Why is an industrial high-temperature muffle furnace necessary for preparing metal oxide/honeycomb catalysts?
- What advancements have been made in muffle furnace technology? Discover Precision and Efficiency Innovations
- What is the operating principle of a muffle furnace? Unlock Pure, Controlled Heating for Your Lab
- What are the main benefits of using a muffle furnace? Achieve Precise, Contamination-Free Heating for Your Lab
- What are the key features of Controlled Atmosphere Muffle Furnaces for brazing? Achieve Strong, Reliable Joints
- What role do muffle furnaces play in the pretreatment of medical samples? Essential for Accurate Elemental Analysis
- What optional features are available for box furnaces? Customize for Your Lab's Unique Needs
- What is the role of a laboratory high-temperature muffle furnace in the pretreatment of peat clay? Unlock Reactivity



















