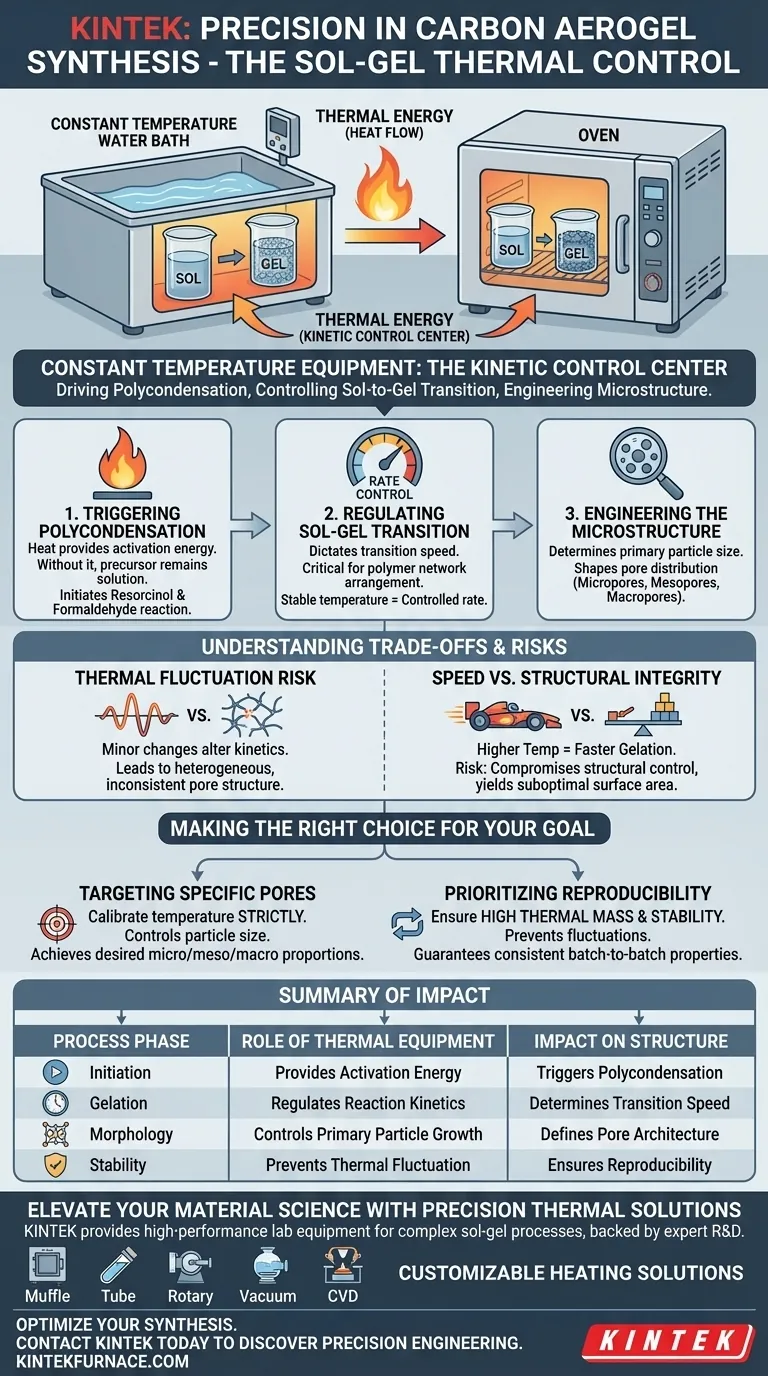
Constant temperature water baths or ovens act as the kinetic control center during the synthesis of phenolic resin-based carbon aerogels. These devices provide the stable thermal environment necessary to trigger and sustain the polycondensation reaction between resorcinol and formaldehyde, physically driving the transition from a liquid precursor (sol) to a solid network (gel).
Precision is the defining factor in this process. By strictly controlling the thermal environment, you determine the rate of reaction and the size of primary particles, which is the primary mechanism for engineering the material's final pore structure.

The Mechanics of Structural Control
Triggering Polycondensation
The fundamental role of this equipment is to initiate the chemical reaction. The heat supplied by the water bath or oven provides the energy required for resorcinol and formaldehyde to engage in polycondensation.
Without this sustained thermal input, the mixture would remain a precursor solution rather than evolving into a cross-linked network.
regulating the Sol-Gel Transition
Beyond simply starting the reaction, constant temperature dictates the speed of the transition. The equipment ensures that the shift from sol to gel occurs at a specific, controlled rate.
This rate control is critical because the speed of gelation directly impacts the physical arrangement of the polymer network.
Engineering the Microstructure
Determining Particle Size
The thermal environment is directly responsible for the size of the primary particles formed during synthesis.
A stable, precise temperature ensures that these particles grow to intended dimensions rather than forming randomly.
Shaping Pore Distribution
The size of the primary particles dictates the architecture of the resulting void spaces. This influences the final distribution of the aerogel's internal structure.
By manipulating the temperature, you effectively tune the proportions of micropores, mesopores, and macropores in the final product.
Understanding the Trade-offs
The Risk of Thermal Fluctuation
The primary pitfall in this process is thermal instability. Even minor fluctuations in the water bath or oven can alter reaction kinetics mid-process.
This leads to a heterogeneous pore structure, where the distribution of micropores and mesopores deviates from the design specifications.
Speed vs. Structural Integrity
Higher temperatures typically accelerate the reaction rate, leading to faster gelation.
However, prioritizing speed can compromise structural control, potentially resulting in particle sizes that do not yield the desired surface area or pore volume.
Making the Right Choice for Your Goal
To maximize the quality of your carbon aerogels, consider the following based on your specific objectives:
- If your primary focus is specific pore targeting: Calibrate your temperature strictly to control particle size, as this directly dictates whether you achieve micropores, mesopores, or macropores.
- If your primary focus is reproducibility: Ensure your equipment has high thermal mass and stability to prevent fluctuations that lead to inconsistent batch-to-batch structural properties.
Mastering the thermal environment is not just about heating; it is about architectural control at the nanoscale.
Summary Table:
| Process Phase | Role of Thermal Equipment | Impact on Material Structure |
|---|---|---|
| Initiation | Provides activation energy | Triggers resorcinol-formaldehyde polycondensation |
| Gelation | Regulates reaction kinetics | Determines speed of sol-to-gel transition |
| Morphology | Controls primary particle growth | Defines final micropore and mesopore architecture |
| Stability | Prevents thermal fluctuation | Ensures batch-to-batch reproducibility and homogeneity |
Elevate Your Material Science with Precision Thermal Solutions
Precise architectural control at the nanoscale requires equipment that delivers absolute thermal stability. KINTEK provides the high-performance lab equipment necessary for complex sol-gel processes, backed by expert R&D and manufacturing.
Whether you require Muffle, Tube, Rotary, Vacuum, or CVD systems, our laboratory high-temperature furnaces and heating solutions are fully customizable to meet your unique research needs.
Ready to optimize your carbon aerogel synthesis? Contact KINTEK today to discover how our precision engineering can enhance your laboratory's efficiency and material integrity.
Visual Guide
References
- Yong Zhong, Xuguang Liu. Carbon Aerogel for Aqueous Phase Adsorption/Absorption: Application Performances, Intrinsic Characteristics, and Regulatory Constructions. DOI: 10.1002/sstr.202400650
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
People Also Ask
- What is the role of high-pressure inert gases in the HPB process? Mastering CZT Crystal Stoichiometry
- What is the graphite furnace technique? A Guide to Ultra-Trace Metal Analysis
- Why is a forced air drying oven essential after molding biomass briquettes? Enhance Fuel Quality & Strength
- What are the primary advantages of industrial microwave heating equipment? Enhanced Uranium Recovery Through Innovation
- What are the advantages of using multi-stage laboratory sintering furnaces? Ensure Defect-Free Powder Metallurgy
- Why is the preheating zone of a walking-beam furnace critical for Titanium/Steel clad plates? Minimize Thermal Stress
- What is the role of carbonaceous reducing agents in copper slag treatment? Maximize Metal Recovery with Expert Insights
- What is the role of sintering in CsPbBr3-SiO2 preparation? Unlock Ultra-Stability with Precise Thermal Sealing



















